Figure 3.
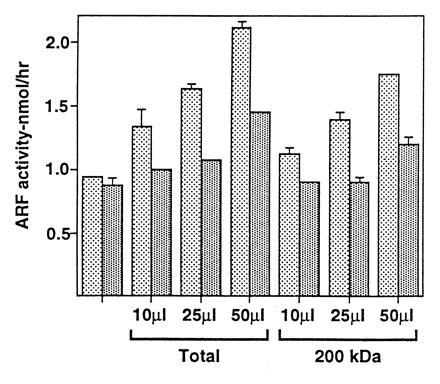
Activity of BFA-inhibited GEP eluted from gel after SDS/PAGE. A sample (≈100 units) of pH 5.8 precipitate was treated with SDS sample buffer at room temperature for 30 min before separation of proteins by SDS/PAGE (4% gel). After electrophoresis, the segment of gel containing protein of ≈200 kDa (referring to position of prestained marker myosin) was excised. Protein was electroeluted for 3 hr in buffer containing 0.1% SDS, followed by electrodialysis for 3 hr in the same buffer without SDS and then dialyzed against buffer B. Another sample of ≈100 units (Total) was treated the same way except that electrophoresis was stopped and elution carried out just before proteins entered the separation gel. GEP activities of indicated amounts of the total and 200-kDa protein fractions are shown. The first of each pair of columns is activity without BFA and the second with 6 μg of BFA.
