Abstract
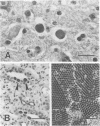
Simian virus 40 (SV40) was isolated from the brains of three rhesus monkeys and the kidneys of two other rhesus monkeys with simian immunodeficiency virus-induced immunodeficiency. A striking feature of these five cases was the tissue specificity of the SV40 replication. SV40 was also isolated from the kidney of a Taiwanese rock macaque with immunodeficiency probably caused by type D retrovirus infection. Multiple full-length clones were derived from all six fresh SV40 isolates, and two separate regions of their genomes were sequenced: the origin (ori)-enhancer region and the coding region for the carboxy terminus of T antigen (T-ag). None of the 23 clones analyzed had two 72-bp enhancer elements as are present in the commonly used laboratory strain 776 of SV40; 22 of these 23 clones were identical in their ori-enhancer sequences, and these had only a single 72-bp enhancer element. We found no evidence for differences in ori-enhancer sequences associated with tissue-specific SV40 replication. The T-ag coding sequence that was analyzed was identical in all clones from kidney. However, significant variation was observed in the carboxy-terminal region of T-ag in SV40 isolated from brain tissues. This sequence variation was located in a region previously reported to be responsible for SV40 host range in cultured cell lines. Thus, SV40 appears to be an opportunistic pathogen in the setting of simian immunodeficiency virus-induced immunodeficiency, similarly to JC virus in human immunodeficiency virus-infected humans, the enhancer sequence organization generally attributed to SV40 is not representative of natural SV40 isolates, and sequence variation near the carboxy terminus of T-ag may play a role in tissue-specific replication of SV40.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Berger J. R., Kaszovitz B., Post M. J., Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med. 1987 Jul;107(1):78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C. The GenBank genetic sequence data bank. Nucleic Acids Res. 1988 Mar 11;16(5):1861–1863. doi: 10.1093/nar/16.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. R., Lees-Miller S. P., Tegtmeyer P., Anderson C. W. The human DNA-activated protein kinase phosphorylates simian virus 40 T antigen at amino- and carboxy-terminal sites. J Virol. 1991 Oct;65(10):5131–5140. doi: 10.1128/jvi.65.10.5131-5140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., BERKELEY W. H., YOUNG R. D. Tumors induced in hamsters by injection of rhesus monkey kidney cell extracts. Proc Soc Exp Biol Med. 1961 May;107:191–197. doi: 10.3181/00379727-107-26576. [DOI] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., GRUBBS G. E., YOUNG R. D. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology. 1962 May;17:65–75. doi: 10.1016/0042-6822(62)90082-x. [DOI] [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992 Mar;66(3):1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum L., Hinrichs S. H., Jay G. JC virus and simian virus 40 enhancers and transforming proteins: role in determining tissue specificity and pathogenicity in transgenic mice. J Virol. 1992 Feb;66(2):1176–1182. doi: 10.1128/jvi.66.2.1176-1182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. J., Gruda M. C., Manuppello J. R., Alwine J. C. Activity of simian DNA-binding factors is altered in the presence of simian virus 40 (SV40) early proteins: characterization of factors binding to elements involved in activation of the SV40 late promoter. J Virol. 1990 Jan;64(1):173–184. doi: 10.1128/jvi.64.1.173-184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., GAYLORD W. H., Jr The vacuolating virus of monkeys. I. Isolation, growth characteristics, and inclusion body formation. J Exp Med. 1961 Dec 1;114:975–986. doi: 10.1084/jem.114.6.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Borden E. C., McBain J. A., Padgett B. L., Walker D. L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980 Mar;92(3):373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Holmberg C. A., Gribble D. H., Takemoto K. K., Howley P. M., Espana C., Osburn B. I. Isolation of simian virus 40 from rhesus monkeys (Macaca mulatta) with spontaneous progressive multifocal leukoencephalopathy. J Infect Dis. 1977 Oct;136(4):593–596. doi: 10.1093/infdis/136.4.593. [DOI] [PubMed] [Google Scholar]
- Horvath C. J., Simon M. A., Bergsagel D. J., Pauley D. R., King N. W., Garcea R. L., Ringler D. J. Simian virus 40-induced disease in rhesus monkeys with simian acquired immunodeficiency syndrome. Am J Pathol. 1992 Jun;140(6):1431–1440. [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Khalili K., Brady J., Pipas J. M., Spence S. L., Sadofsky M., Khoury G. Carboxyl-terminal mutants of the large tumor antigen of simian virus 40: a role for the early protein late in the lytic cycle. Proc Natl Acad Sci U S A. 1988 Jan;85(2):354–358. doi: 10.1073/pnas.85.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N. W., Hunt R. D., Letvin N. L. Histopathologic changes in macaques with an acquired immunodeficiency syndrome (AIDS). Am J Pathol. 1983 Dec;113(3):382–388. [PMC free article] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz R. B., Eaton B. A., Kubik M. F., Latorra D., McGregor J. A., Dynan W. S. BK virus and JC virus shed during pregnancy have predominantly archetypal regulatory regions. J Virol. 1991 Aug;65(8):4515–4519. doi: 10.1128/jvi.65.8.4515-4519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., Li P. N. Comparison of regulatory sequences and enhancer activities of SV40 variants isolated from patients with neurological diseases. Virus Res. 1991 May;19(2-3):163–172. doi: 10.1016/0168-1702(91)90043-u. [DOI] [PubMed] [Google Scholar]
- Martin J. D. Regulatory sequences of SV40 variants isolated from patients with progressive multifocal leukoencephalopathy. Virus Res. 1989 Sep;14(1):85–94. doi: 10.1016/0168-1702(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Munholland J. M., Kelly J. J., Hassell J. A., Wildeman A. G. Cell specificity of transcription regulation by papovavirus T antigens and DNA replication. EMBO J. 1992 Jan;11(1):177–184. doi: 10.1002/j.1460-2075.1992.tb05040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Pipas J. M. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J Virol. 1985 May;54(2):569–575. doi: 10.1128/jvi.54.2.569-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rosen S., Harmon W., Krensky A. M., Edelson P. J., Padgett B. L., Grinnell B. W., Rubino M. J., Walker D. L. Tubulo-interstitial nephritis associated with polyomavirus (BK type) infection. N Engl J Med. 1983 May 19;308(20):1192–1196. doi: 10.1056/NEJM198305193082004. [DOI] [PubMed] [Google Scholar]
- Rosenthal N., Kress M., Gruss P., Khoury G. BK viral enhancer element and a human cellular homolog. Science. 1983 Nov 18;222(4625):749–755. doi: 10.1126/science.6314501. [DOI] [PubMed] [Google Scholar]
- Rubinstein R., Schoonakker B. C., Harley E. H. Recurring theme of changes in the transcriptional control region of BK virus during adaptation to cell culture. J Virol. 1991 Mar;65(3):1600–1604. doi: 10.1128/jvi.65.3.1600-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr, Narayan O., Danna K. J., Weiner L. P., Nathans D. The nucleic acid of an SV40-like virus isolated from a patient with progressive multifocal leukoencephalopathy. Virology. 1973 Feb;51(2):345–350. doi: 10.1016/0042-6822(73)90433-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Buck M., Schneider J., Kalderon D., Fanning E., Smith A. E. Biochemical characterization of phosphorylation site mutants of simian virus 40 large T antigen: evidence for interaction between amino- and carboxy-terminal domains. J Virol. 1991 Mar;65(3):1479–1490. doi: 10.1128/jvi.65.3.1479-1490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy T., Chamberlain M., Cole C. N. Simian virus 40 host range/helper function mutations cause multiple defects in viral late gene expression. J Virol. 1989 Dec;63(12):5208–5215. doi: 10.1128/jvi.63.12.5208-5215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavis J. E., Walker D. L., Gardner S. D., Frisque R. J. Nucleotide sequence of the human polyomavirus AS virus, an antigenic variant of BK virus. J Virol. 1989 Feb;63(2):901–911. doi: 10.1128/jvi.63.2.901-911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T., Yogo Y., Kitamura T., Aso Y. Persistence of archetypal JC virus DNA in normal renal tissue derived from tumor-bearing patients. Virology. 1992 Feb;186(2):736–741. doi: 10.1016/0042-6822(92)90040-v. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. doi: 10.1111/j.1432-1033.1979.tb02032.x. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Herndon R. M., Narayan O., Johnson R. T., Shah K., Rubinstein L. J., Preziosi T. J., Conley F. K. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N Engl J Med. 1972 Feb 24;286(8):385–390. doi: 10.1056/NEJM197202242860801. [DOI] [PubMed] [Google Scholar]