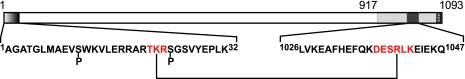
Fig. 5.
Domain map of the β subunit of PhK showing regions of intramolecular cross-linking by GMBS. Seryl residues within the N-terminal region that are phosphorylated by either cAMP-dependent protein kinase or PhK autophosphorylation are indicated by a P below them. The N-terminal 32 residues of β, indicated by gradation from white to black, represent a region of the subunit that has been shown to regulate homodimeric β interactions in two-hybrid screens (23). A region required for homodimeric interactions of β in two-hybrid screens corresponds to residues 917–1093 (light gray) and includes a stretch of residues (1026–1047) that are a predicted to have high propensity for forming a coiled-coil domain (23). GMBS intramolecular cross-linking of these two regions in the β subunit of the activated PhK complex is indicated by peptides (shown in red lettering) cross-linked through residues Lys-22 and Arg-1040.