Figure 5.
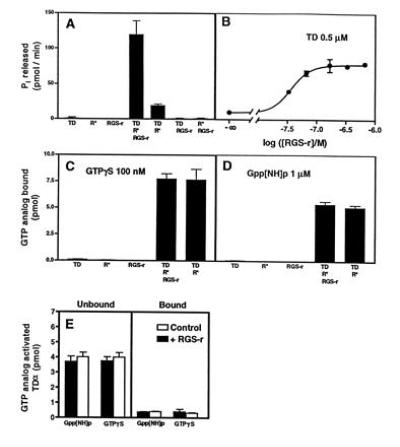
Enhanced GTP hydrolysis of TDα by mRGS-r protein without affecting the loading of GTP. (A) mRGS-r protein increases the Pi release from TDα. The hydrolysis of GTP (10 μM) by 0.5 μM TD, 10 μg of urea-treated ROS membranes (R*, bleached rhodopsin in rod outer segment membranes), 0.7 μM mRGS-r, or combinations of these components was measured for 3 min at 30°C. Hydrolysis of GTP was observed in the presence of R* and TD. mRGS-r protein increased the Pi release from TD ≈7-fold. (B) Dose–response curve of mRGS-r protein. Half-maximal and maximal stimulation by mRGS-r protein was observed at 50 and 200 nM, respectively. (C and D) The binding of nonhydrolyzable GTP analogs to TD was not affected by mRGS-r protein. GTP[γS] (100 nM) or Gpp[NH]p (1 μM) was used as described. (E) TD activated by nonhydrolyzable GTP analogs does not bind to mRGS-r protein. TD loaded with labeled nonhydrolyzable GTP analogs was prepared by centrifugation of the reaction mixtures from C and D. The supernatants, containing the labeled TD and mRGS-r protein, were applied to Ni2+-NTA columns. The amount of protein-bound radioactivity in the flow-through and the 400 mM imidazole eluate was determined. In all parts of this figure, means ± SD of triplicate determinations are shown.
