Figure 1.
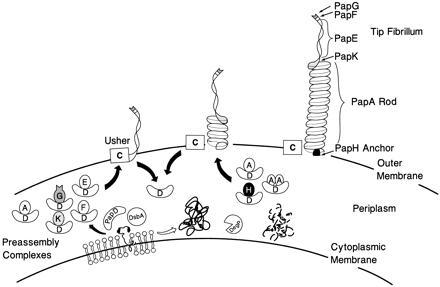
Model of pilus assembly. Details of the model are discussed in the text. PapD has an immunoglobulin-like three-dimensional structure (9) and is the prototype member of a large family of PapD-like chaperones required for pilus assembly in Gram-negative bacteria (13). DsbA mediates disulfide bond formation in PapD and in the pilus subunits (14). DsbA is required for the correct folding of PapD. Closed arrow depicts pathway subunits travel in the presence of PapD chaperone. Subunits misfold and are proteolytically degraded by the DegP protease in the absence of an interaction with PapD (open arrow) (C.H.J. and S.J.H., unpublished work). Chaperone–subunit complexes are targeted to the PapC usher protein (12) where pilus assembly occurs; an essential step in assembly is the displacement of PapD (uncapping). The PapD chaperone has been shown to interact with pilus subunits, in part, via the COOH terminal motif (15). Binding of PapD to an assembly surface on the subunits modulates the formation of the pilus rod.
