Figure 2.
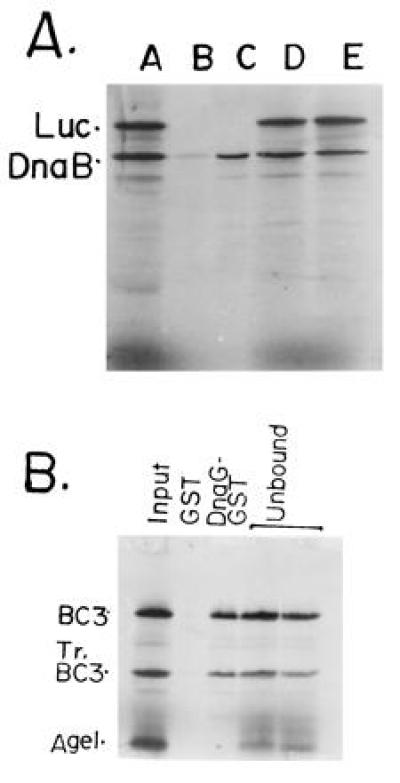
GST-affinity chromatography showing the binding of full-length DnaB and its peptides with DnaG-GST fusion protein affinity matrix. (A) Full-length DnaB and luciferase (Luc; negative control) were labeled with 35S-methionine by coupled in vitro transcription-translation in rabbit reticulocyte extracts. An approximately equimolar mixture of the two labeled proteins were loaded on to the control GST-glutathione-agarose and the DnaG-GST-glutathione-agarose matrices. The flow through fraction was collected and analyzed. The beads were washed and the proteins eluted and resolved by SDS/PAGE. An autoradiogram of the gel shows: lane 1, input mixture of full-length DnaB, a truncated product of DnaB (N-terminal fragment) and full-length luciferase (Luc); lane 2, protein bound to the control GST-agarose matrix; lane 3, protein bound to the DnaG-GST-agarose matrix. Note that full-length DnaB, its truncated peptide, that was generated during the labeling process, were selectively retained on the DnaG-GST matrix but not on the control GST-matrix. No luciferase bound to the control or to the DnaG-GST matrices. (B) DNA template encoding the BC3 and AgeI (see Fig. 3) peptides of DnaB were labeled in vitro as described. The In vitro labeling process generated the tr.BC3 (Tr.BC3) peptide. The truncation must have generated a N-terminal peptide because of the upstream location of the T7 promoter used to transcribe the DNA. Lanes: 1, input mixture of BC3, TrBC3 and the AgeI peptides; 2, binding to the control GST matrix; 3, binding to the DnaG-GST matrix; 4, flow through from the GST matrix; 5, flow through from the DnaG-GST matrix. Note that the BC3 and tr.BC3 peptides (≈40–50% of the input) were retained on the DnaG-GST but not on the control GST matrix. The AgeI peptide was not retained by either matrices and was recovered in the flow through fractions.
