Abstract
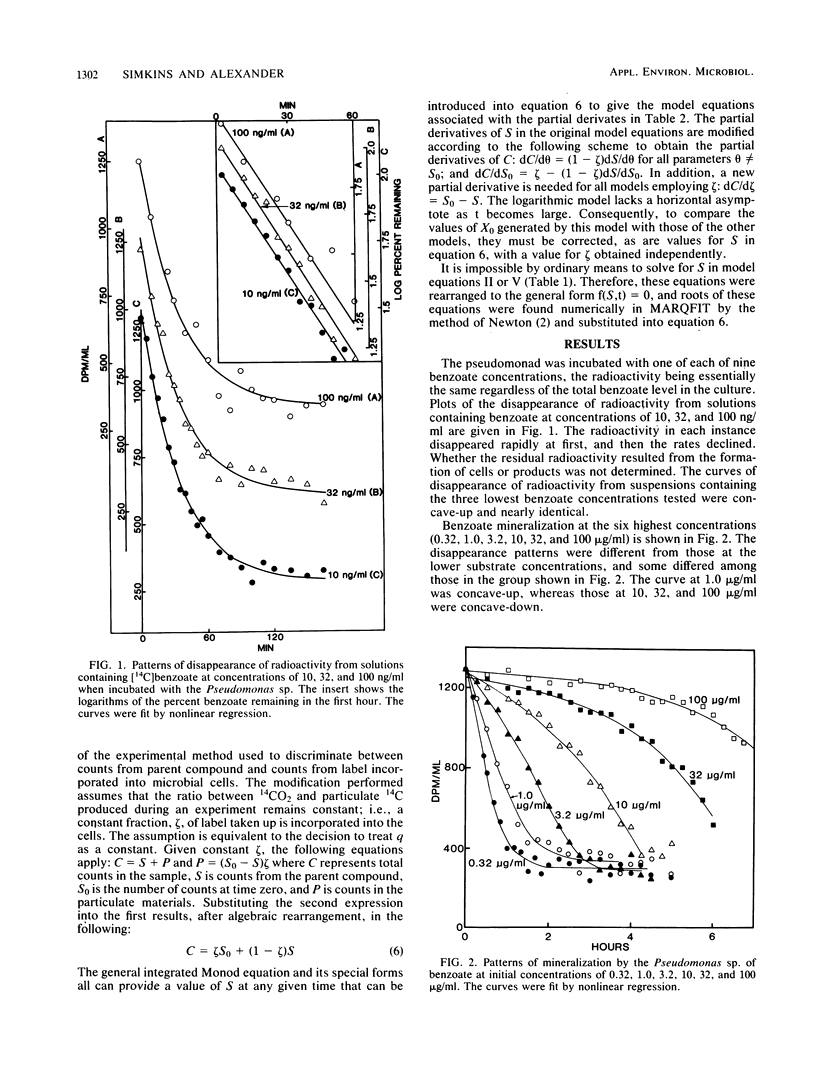
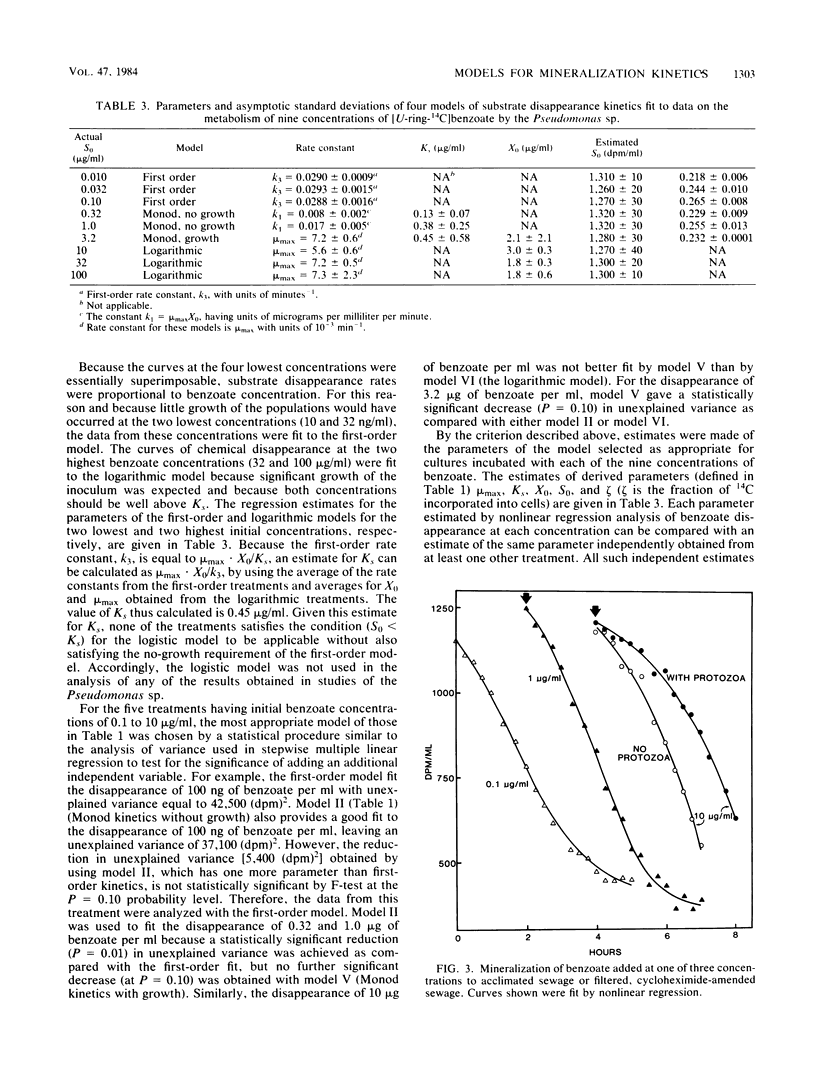
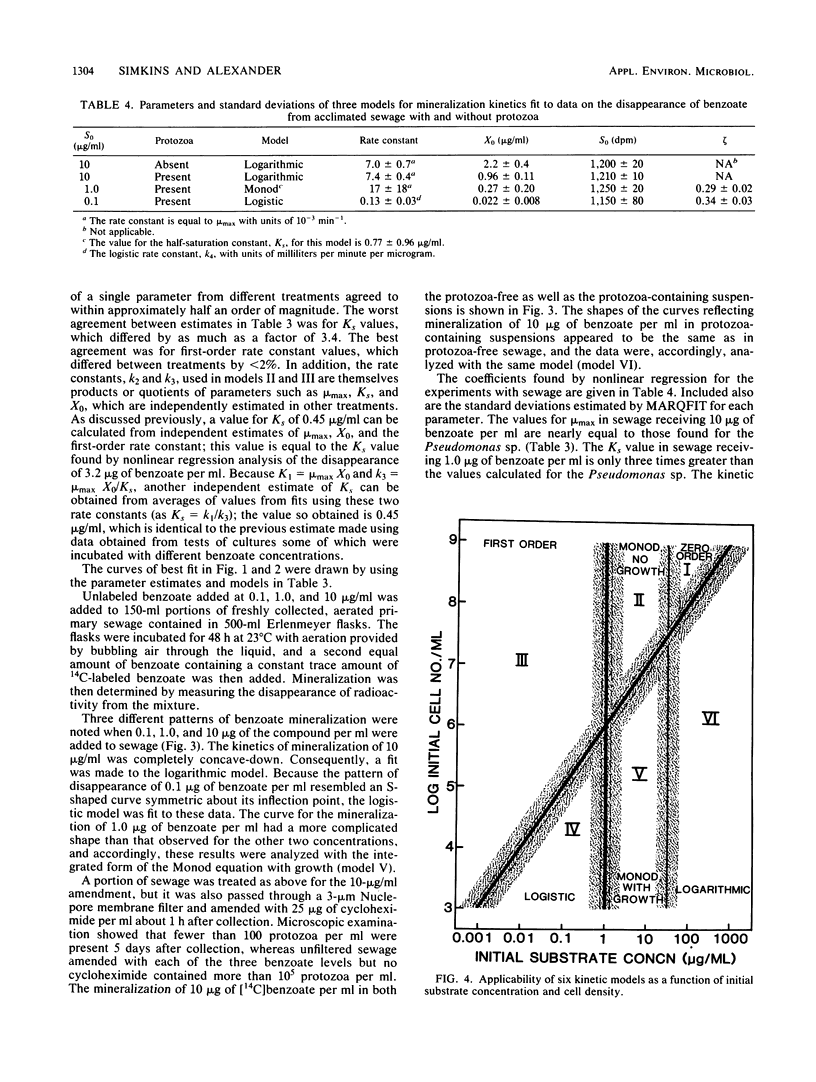
The rates of mineralization of [14C]benzoate by an induced population of Pseudomonas sp. were measured at initial substrate concentrations ranging from 10 ng/ml to 100 micrograms/ml. Plots of the radioactivity remaining in the culture were fit by nonlinear regression to six kinetic models derived from the Monod equation. These models incorporate only the variables of substrate concentration and cell density. Plots of the mineralization kinetics in cultures containing low, intermediate, and high initial substrate concentrations were well fit by first-order, integrated Monod, and logarithmic kinetics, respectively. Parameters such as maximum specific growth rate, half-saturation constant, and initial population density divided by yield agreed between cultures to within a factor of 3.4. Benzoate mineralization by microorganisms in acclimated sewage was shown to fit logistic (sigmoidal), Monod, and logarithmic kinetics when the compound was added at initial concentrations of 0.1, 1.0, and 10 micrograms/ml, respectively. The mineralization of 10 micrograms of benzoate per ml in sewage also followed logarithmic kinetics in the absence of protozoa. It is concluded that much of the diversity in shapes of mineralization curves is a result of the interactions of substrate concentration and population density. Nonlinear regression with models incorporating these variables is a valuable means for analysis of microbial mineralization kinetics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Counotte G. H., Prins R. A. Calculation of k and v from substrate concentration versus time plot. Appl Environ Microbiol. 1979 Oct;38(4):758–760. doi: 10.1128/aem.38.4.758-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Loveless J. E. The effect of growth conditions on respiratory activity and growth efficiency in facultative anaerobes grown in chemostat culture. J Gen Microbiol. 1971 Sep;68(1):35–43. doi: 10.1099/00221287-68-1-35. [DOI] [PubMed] [Google Scholar]
- Huang T. C., Chang M. C., Alexander M. Effect of protozoa on bacterial degradation of an aromatic compound. Appl Environ Microbiol. 1981 Jan;41(1):229–232. doi: 10.1128/aem.41.1.229-232.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Multistep kinetics: choice of models for the growth of bacteria. J Theor Biol. 1982 Oct 7;98(3):401–417. doi: 10.1016/0022-5193(82)90127-8. [DOI] [PubMed] [Google Scholar]
- Mateles R. K., Ryu D. Y., Yasuda T. Measurement of unsteady state growth rates of micro-organisms. Nature. 1965 Oct 16;208(5007):263–265. doi: 10.1038/208263a0. [DOI] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. R., Button D. K. Phosphate-limited continuous culture of Rhodotorula rubra: kinetics of transport, leakage, and growth. J Bacteriol. 1979 Jun;138(3):884–895. doi: 10.1128/jb.138.3.884-895.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Nonlinear estimation of Monod growth kinetic parameters from a single substrate depletion curve. Appl Environ Microbiol. 1983 May;45(5):1453–1458. doi: 10.1128/aem.45.5.1453-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. L., McClellan J. F., Coleman D. C. Nitrogen Mineralization by Acanthamoeba polyphaga in Grazed Pseudomonas paucimobilis Populations. Appl Environ Microbiol. 1981 Oct;42(4):667–671. doi: 10.1128/aem.42.4.667-671.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smouse P. E. Mathematical models for continuous culture growth dynamics of mixed populations subsisting on a heterogeneous resource base: I. Simple competition. Theor Popul Biol. 1980 Feb;17(1):16–36. doi: 10.1016/0040-5809(80)90012-x. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Subba-Rao R. V., Alexander M. Effect of sorption on mineralization of low concentrations of aromatic compounds in lake water samples. Appl Environ Microbiol. 1982 Sep;44(3):659–668. doi: 10.1128/aem.44.3.659-668.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordy J. R., Alexander M. Microbial metabolism of N-nitrosodiethanolamine in lake water and sewage. Appl Environ Microbiol. 1980 Mar;39(3):559–565. doi: 10.1128/aem.39.3.559-565.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


