Abstract
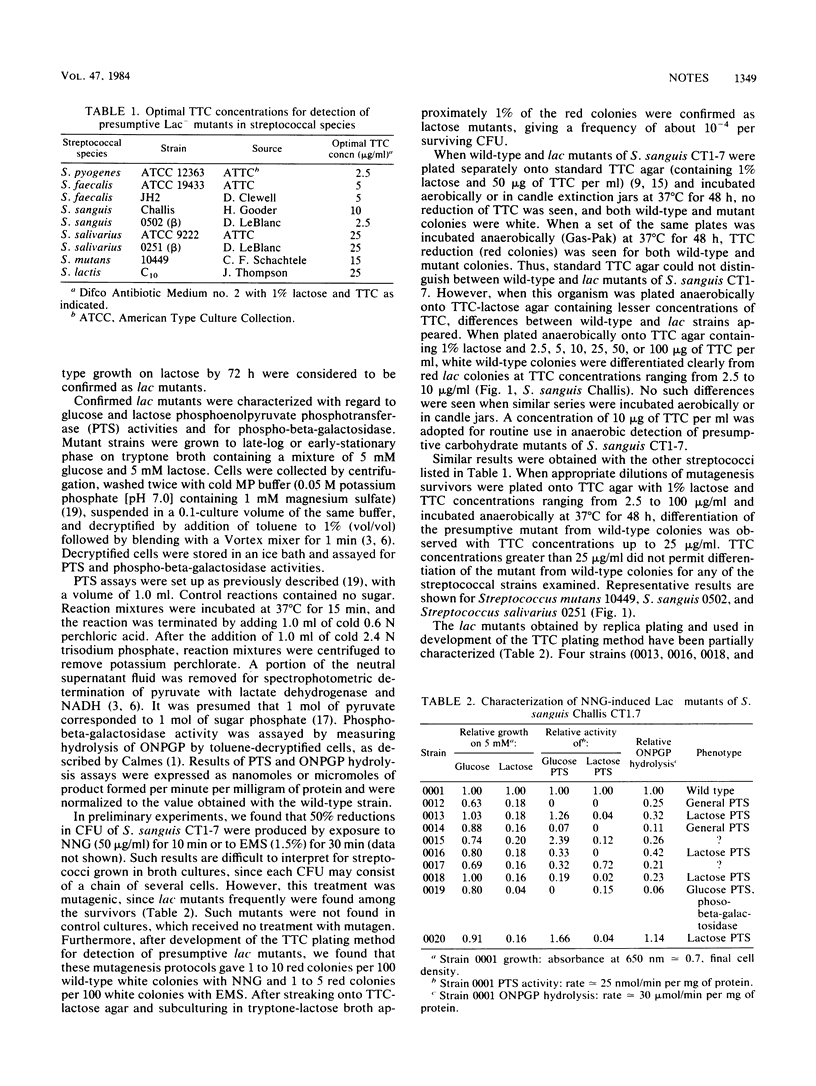
The tetrazolium method for detection of bacterial mutants defective in sugar catabolism was modified for use with streptococci. The critical factors were (i) the concentration of tetrazolium, which must be titrated to determine the optimum concentration for each species or even strain, and (ii) anaerobic incubation of tetrazolium-containing agar plates. When used with standard mutagenesis protocols, this method yielded lactose-negative mutants of nine streptococcal strains representing six species. A collection of lactose-negative mutants of streptococcus, sanguis Challis was characterized and contained phospho-beta-galactosidase, lactose phosphotransferase, and general phosphotransferase mutants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. R., Jr, Blevins W. T., Feary T. W. Interspecies transformation of streptomycin resistance in oral streptococci. Antimicrob Agents Chemother. 1976 Jan;9(1):145–150. doi: 10.1128/aac.9.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D. Lactate dehydrogenase mutants of Streptococcus mutans: isolation and preliminary characterization. Infect Immun. 1978 Jul;21(1):206–212. doi: 10.1128/iai.21.1.206-212.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hawley R. J., Lee L. N., St Martin E. J. "Conjugal" transfer of plasmid DNA among oral streptococci. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3484–3487. doi: 10.1073/pnas.75.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Detection of Fermentative Variants with Tetrazolium. J Bacteriol. 1948 Nov;56(5):695–695. doi: 10.1128/jb.56.5.695-695.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuhl G. H., Gooder H. Biochemistry and genetics of galactose metabolism in group H Streptococcus strain Challis. J Bacteriol. 1972 Feb;109(2):512–519. doi: 10.1128/jb.109.2.512-519.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Genetic transformation of Streptococcus sanguis (Challis) with cryptic plasmids from Streptococcus ferus. Infect Immun. 1980 Jun;28(3):692–699. doi: 10.1128/iai.28.3.692-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants defective in adherence and aggregation. Infect Immun. 1981 Dec;34(3):1044–1055. doi: 10.1128/iai.34.3.1044-1055.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Germaine G. R., Harlander S. K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans. Infect Immun. 1975 Oct;12(4):934–937. doi: 10.1128/iai.12.4.934-937.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Westergren G., Emilson C. G. Transformation of streptococci to streptomycin resistance by oral streptococcal DNA. Arch Oral Biol. 1977;22(8-9):533–537. doi: 10.1016/0003-9969(77)90051-6. [DOI] [PubMed] [Google Scholar]
- Whittenbury R. Biochemical characteristics of Streptococcus species. Soc Appl Bacteriol Symp Ser. 1978;7:51–69. [PubMed] [Google Scholar]




