Abstract
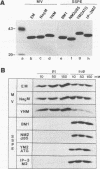
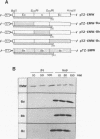
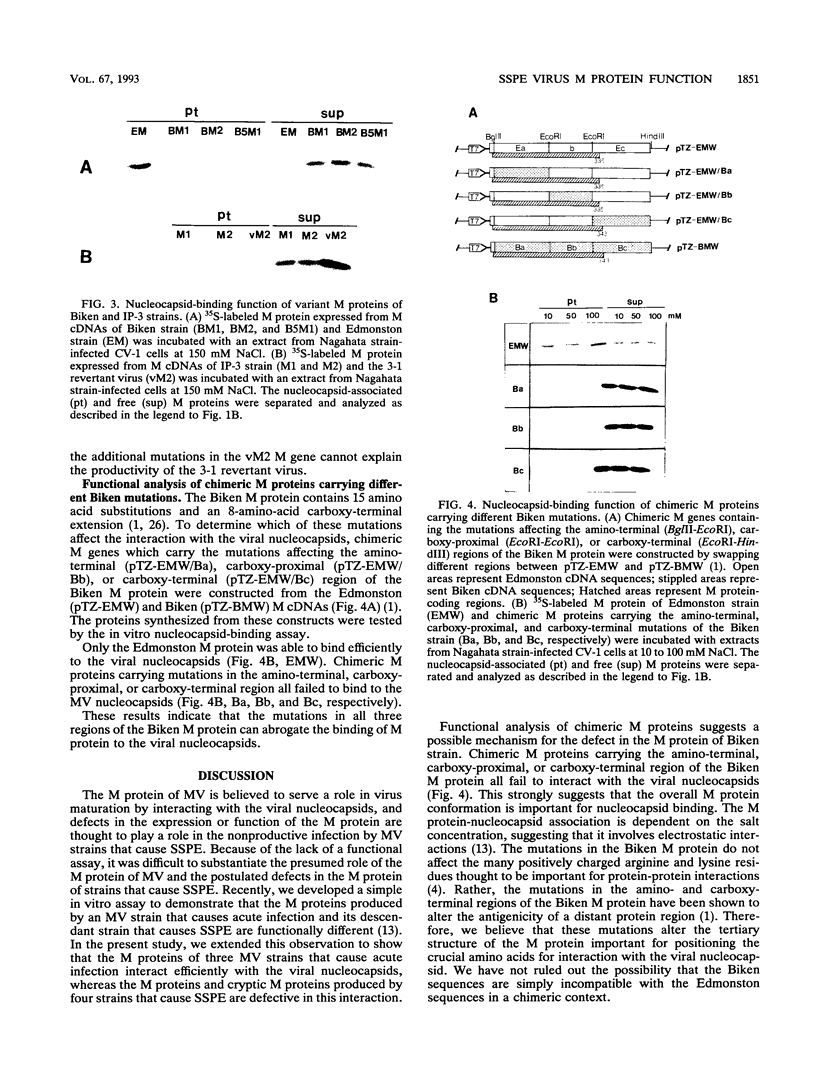
We have developed an in vitro nucleocapsid-binding assay for studying the function of the matrix (M) protein of measles virus (MV) (A. Hirano, A. H. Wang, A. F. Gombart, and T. C. Wong, Proc. Natl. Acad. Sci. USA, 89:8745-8749, 1992). In this communication we show that the M proteins of three MV strains that cause acute infection (Nagahata, Edmonston, and YN) bind efficiently to the viral nucleocapsids whereas the M proteins of four MV strains isolated from patients with subacute sclerosing panencephalitis (SSPE) (Biken, IP-3, Niigata, and Yamagata) fail to bind to the viral nucleocapsids. MV Biken (an SSPE-related virus) produces variant M sequences which encode two antigenically distinct forms of M protein. A serine-versus-leucine difference is responsible for the antigenic variation. MV IP-3 (an SSPE-related virus) also produces variant M sequences, some of which have been postulated to encode a functional M protein responsible for the production of an infectious revertant virus. However, the variant M proteins of Biken and IP-3 strains show no nucleocapsid-binding activity. These results demonstrate that the nucleocapsid-binding function is conserved in the M proteins of MV strains that cause acute infection and that the M proteins of MV strains that cause SSPE exhibit a common defect in this function. Analysis of chimeric M proteins indicates that mutations in the amino-terminal, carboxy-proximal, or carboxy-terminal region of the M protein all abrogate nucleocapsid binding, suggesting that the M protein conformation is important for interaction with the viral nucleocapsid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayata M., Hirano A., Wong T. C. Altered translation of the matrix genes in Niigata and Yamagata neurovirulent measles virus strains. Virology. 1991 Jan;180(1):166–174. doi: 10.1016/0042-6822(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S., Lazzarini R. A. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986 May;58(2):408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Measles virus and chronic neurological diseases. Ann Neurol. 1981 Jan;9(1):17–20. doi: 10.1002/ana.410090104. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A. Subacute sclerosing panencephalitis virus dominantly interferes with replication of wild-type measles virus in a mixed infection: implication for viral persistence. J Virol. 1992 Apr;66(4):1891–1898. doi: 10.1128/jvi.66.4.1891-1898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Wang A. H., Gombart A. F., Wong T. C. The matrix proteins of neurovirulent subacute sclerosing panencephalitis virus and its acute measles virus progenitor are functionally different. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8745–8749. doi: 10.1073/pnas.89.18.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Expression of five viral antigens in cells infected with wild-type and SSPE strains of measles virus: correlation with cytopathic effects and productivity of infections. Arch Virol. 1982;73(3-4):255–262. doi: 10.1007/BF01318079. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., Baczko K., Budka H., ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986 Nov;67(Pt 11):2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Absence of M protein in a cell-associated subacute sclerosing panencephalitis virus. Nature. 1980 Jun 12;285(5765):490–492. doi: 10.1038/285490a0. [DOI] [PubMed] [Google Scholar]
- Sato T. A., Fukuda A., Sugiura A. Characterization of major structural proteins of measles virus with monoclonal antibodies. J Gen Virol. 1985 Jul;66(Pt 7):1397–1409. doi: 10.1099/0022-1317-66-7-1397. [DOI] [PubMed] [Google Scholar]
- Schmid A., Cattaneo R., Billeter M. A. A procedure for selective full length cDNA cloning of specific RNA species. Nucleic Acids Res. 1987 May 26;15(10):3987–3996. doi: 10.1093/nar/15.10.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Spielhofer P., Cattaneo R., Baczko K., ter Meulen V., Billeter M. A. Subacute sclerosing panencephalitis is typically characterized by alterations in the fusion protein cytoplasmic domain of the persisting measles virus. Virology. 1992 Jun;188(2):910–915. doi: 10.1016/0042-6822(92)90552-z. [DOI] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Rapid degradation restricts measles virus matrix protein expression in a subacute sclerosing panencephalitis cell line. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7913–7917. doi: 10.1073/pnas.83.20.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Okuno Y., Hamamoto Y., Oya H. Subacute sclerosing panencephalitis (SSPE): isolation of a defective variant of measles virus from brain obtained at autopsy. Biken J. 1975 Jun;18(2):113–122. [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- Wechsler S. L., Weiner H. L., Fields B. N. Immune response in subacute sclerosing panencephalitis: reduced antibody response to the matrix protein of measles virus. J Immunol. 1979 Aug;123(2):884–889. [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Ueda S., Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991 May;65(5):2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Hirano A. Functional cDNA library for efficient expression of measles virus-specific gene products in primate cells. J Virol. 1986 Jan;57(1):343–348. doi: 10.1128/jvi.57.1.343-348.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]