Figure 1.
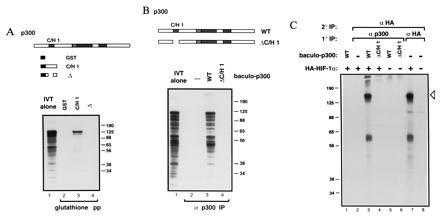
p300 binds HIF-1α (28). (A) GST fusion proteins containing the indicated portions of p300 C/H1 were constructed. 35S-Labeled HIF-1α was transcribed and translated in vitro and mixed with the indicated GST fusion proteins immobilized on glutathione beads. Bead-bound proteins were visualized by SDS/PAGE and autoradiography. (B) Full-length p300 (WT) and p300 deleted within the first cysteine/histidine-rich region (C/H1) were synthesized in insect cells using the baculovirus system. 35S-labeled HIF-1α was synthesized in vitro, and translation products were mixed with the indicated baculo-p300 species and immunoprecipitated with a p300 antibody [RW128 (4)] using protein-A Sepharose beads. Radiolabeled bead-bound proteins were visualized by SDS/PAGE and autoradiography. Lanes 1 in A and B each contain 20% of the input translation products analyzed in the other lanes. (C) U-2 OS cells were transfected with 10 μg of pCMVβ-HA-HIF-1α (+) or vector alone (−). Cells were labeled with [35S]methionine, and cellular extracts were prepared, mixed (where indicated) with the relevant baculovirus-p300 species, and then immunoprecipitated with either anti-p300 or anti-HA antibody, as in B. Bead-bound proteins were released by boiling, and reimmunoprecipitated with antibody to the hemagglutinin (HA) epitope (12CA5). Proteins bound to beads in this second round were visualized by SDS/PAGE and autoradiography. The open arrowhead indicates HA–HIF-1α. The identity of the faster migrating band is not clear but may represent a degradation product. Standard molecular weights (kDa; Sigma) are indicated. IVT, in vitro translate; pp, precipitation; IP, immunoprecipitation.
