Abstract
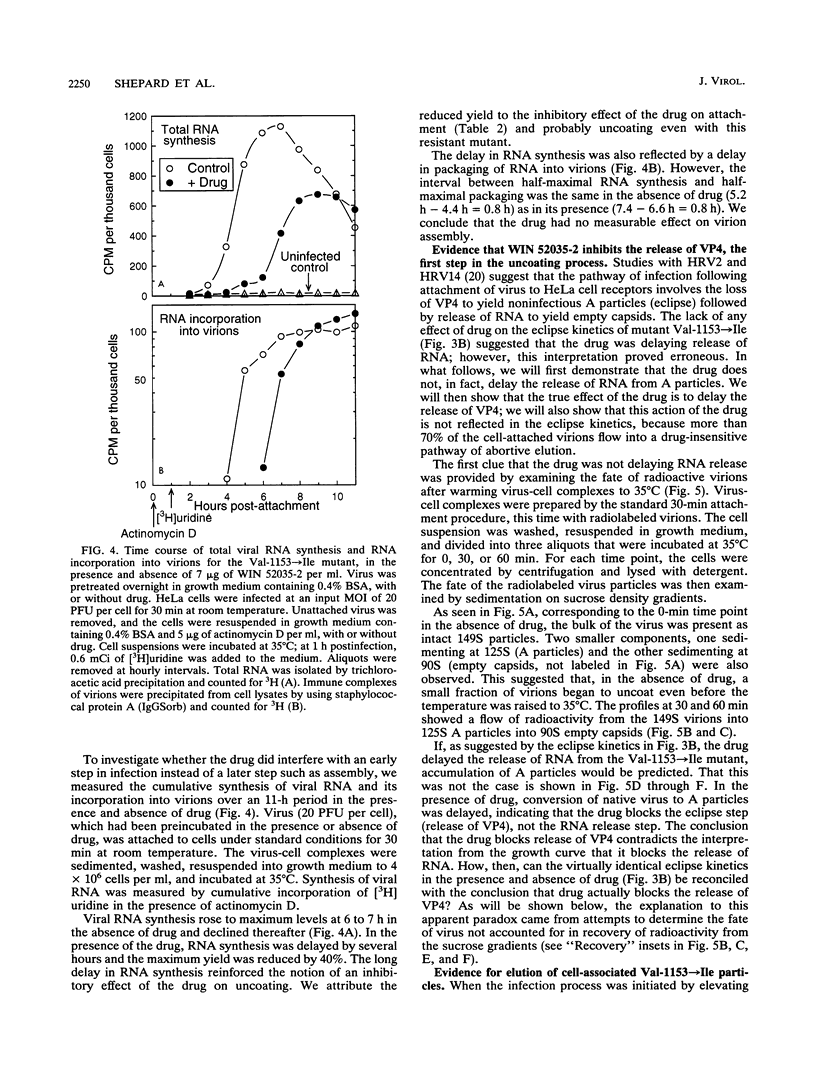
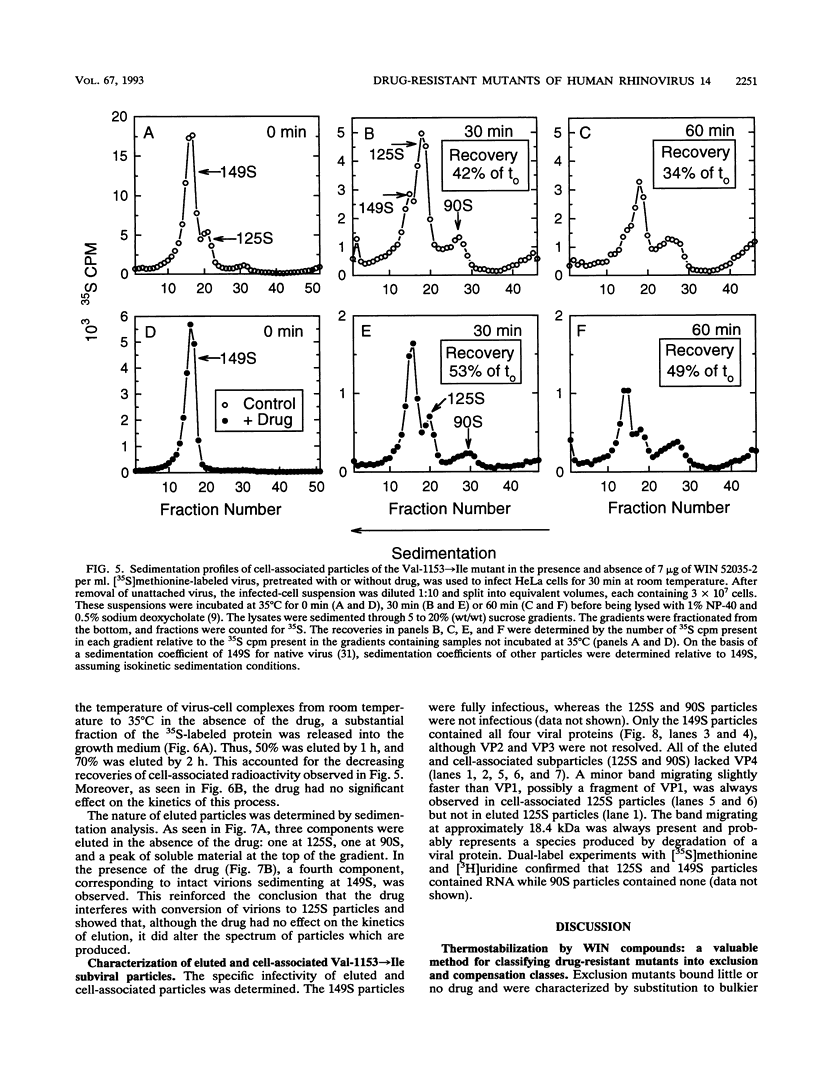
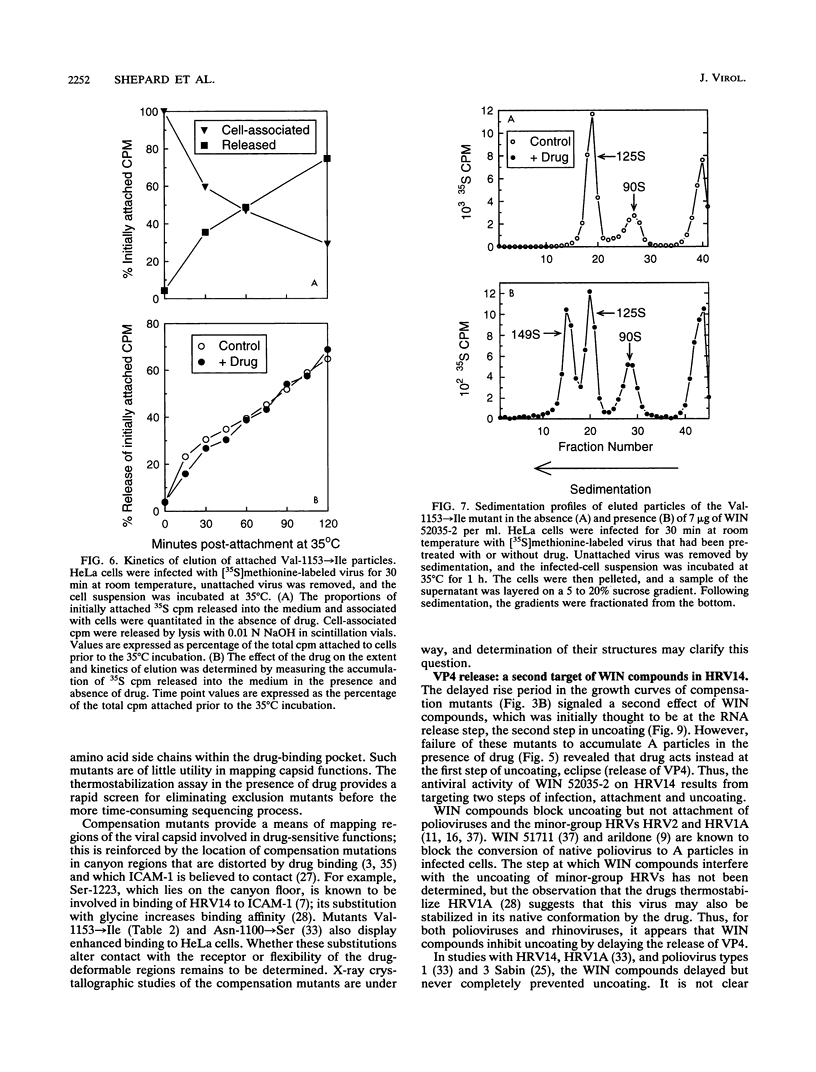
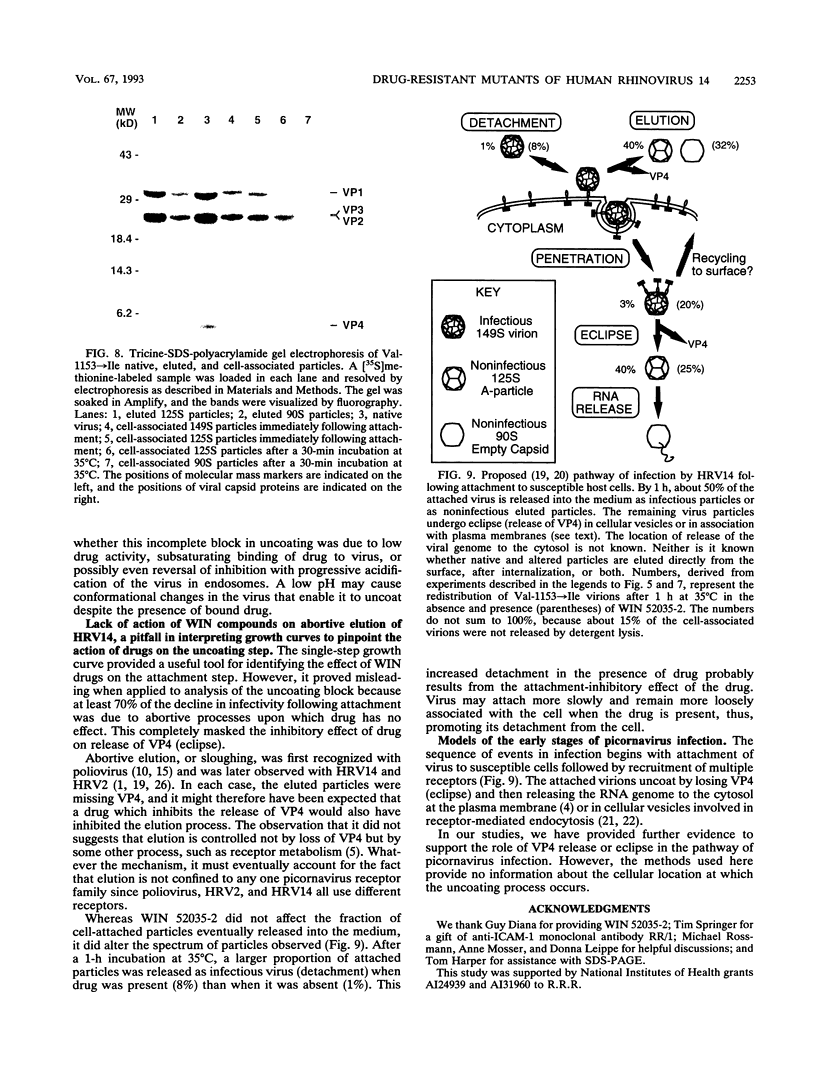
WIN compounds inhibit attachment of human rhinovirus 14 by binding to a hydrophobic pocket within the capsid and inducing conformational changes in the canyon floor, the region that binds the cellular receptor. To study the basis of drug resistance, we isolated and characterized a family of human rhinovirus 14 mutants resistant to WIN 52035-2. Thermostabilization data and single-cycle growth curves provided evidence for two classes of resistant mutants. One class, here called exclusion mutants, showed a marked decrease in drug-binding affinity and was characterized by substitution to bulkier amino acid side chains at two sites lining the hydrophobic pocket. The other class, called compensation mutants, displayed single-amino-acid substitutions in the drug-deformable regions of the canyon; these mutants were able to attach to cells despite the presence of bound drug. A delay in the rise period of the growth curves of compensation mutants indicated a second locus of drug action. WIN 52035-2 was found to inhibit the first step of uncoating, release of VP4. Attempts to identify this site of drug action by using single-step growth curves were obscured by abortive elution of a major fraction of cell-attached virus. The drug had no effect on the rate of this process but did affect the spectrum of particles produced.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Characterization of human rhinoviruses displaced by an anti-receptor monoclonal antibody. J Virol. 1988 Jul;62(7):2300–2306. doi: 10.1128/jvi.62.7.2300-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger J., Krishnaswamy S., Kremer M. J., Oliveira M. A., Rossmann M. G., Heinz B. A., Rueckert R. R., Dutko F. J., McKinlay M. A. Three-dimensional structures of drug-resistant mutants of human rhinovirus 14. J Mol Biol. 1989 May 5;207(1):163–174. doi: 10.1016/0022-2836(89)90447-6. [DOI] [PubMed] [Google Scholar]
- Badger J., Minor I., Kremer M. J., Oliveira M. A., Smith T. J., Griffith J. P., Guerin D. M., Krishnaswamy S., Luo M., Rossmann M. G. Structural analysis of a series of antiviral agents complexed with human rhinovirus 14. Proc Natl Acad Sci U S A. 1988 May;85(10):3304–3308. doi: 10.1073/pnas.85.10.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V. I., Greene M. I. Molecular and cellular mechanisms of receptor-mediated endocytosis. DNA Cell Biol. 1991 Jul-Aug;10(6):399–409. doi: 10.1089/dna.1991.10.399. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., McSharry J. J., Lawrence G. W. Effect of arildone on modifications of poliovirus in vitro. Virology. 1980 Aug;105(1):86–93. doi: 10.1016/0042-6822(80)90158-0. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Condra J. H., Mizutani S., Callahan P. L., Davies M. E., Murcko M. A. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–5453. doi: 10.1073/pnas.85.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers H. J. Selective inhibiton of uncoating of echovirus 12 by rhodanine. A study on early virus-cell interactions. Virology. 1977 May 1;78(1):241–252. doi: 10.1016/0042-6822(77)90095-2. [DOI] [PubMed] [Google Scholar]
- Everaert L., Vrijsen R., Boeyé A. Eclipse products of poliovirus after cold-synchronized infection of HeLa cells. Virology. 1989 Jul;171(1):76–82. doi: 10.1016/0042-6822(89)90512-6. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- Fox M. P., Otto M. J., McKinlay M. A. Prevention of rhinovirus and poliovirus uncoating by WIN 51711, a new antiviral drug. Antimicrob Agents Chemother. 1986 Jul;30(1):110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Heinz B. A., Rueckert R. R., Shepard D. A., Dutko F. J., McKinlay M. A., Fancher M., Rossmann M. G., Badger J., Smith T. J. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J Virol. 1989 Jun;63(6):2476–2485. doi: 10.1128/jvi.63.6.2476-2485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Kim S. S., Smith T. J., Chapman M. S., Rossmann M. C., Pevear D. C., Dutko F. J., Felock P. J., Diana G. D., McKinlay M. A. Crystal structure of human rhinovirus serotype 1A (HRV1A). J Mol Biol. 1989 Nov 5;210(1):91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Noble-Harvey J. Comparison of in vitro and cell-mediated alteration of a human Rhinovirus and its inhibition by sodium dodecyl sulfate. J Virol. 1973 Oct;12(4):819–826. doi: 10.1128/jvi.12.4.819-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984 Apr;98(4):1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Requirements for entry of poliovirus RNA into cells at low pH. EMBO J. 1984 Sep;3(9):1945–1950. doi: 10.1002/j.1460-2075.1984.tb02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Caliguiri L. A., Eggers H. J. Inhibition of uncoating of poliovirus by arildone, a new antiviral drug. Virology. 1979 Sep;97(2):307–315. doi: 10.1016/0042-6822(79)90342-8. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Rueckert R. R. WIN 51711-dependent mutants of poliovirus type 3: evidence that virions decay after release from cells unless drug is present. J Virol. 1993 Mar;67(3):1246–1254. doi: 10.1128/jvi.67.3.1246-1254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J., Lonberg-Holm K. Interactions of components of human rhinovirus type 2 with Hela cells. Virology. 1973 Feb;51(2):270–278. doi: 10.1016/0042-6822(73)90427-3. [DOI] [PubMed] [Google Scholar]
- Olson N. H., Kolatkar P. R., Oliveira M. A., Cheng R. H., Greve J. M., McClelland A., Baker T. S., Rossmann M. G. Structure of a human rhinovirus complexed with its receptor molecule. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):507–511. doi: 10.1073/pnas.90.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Meriam C. Poliovirus temperature-sensitive mutant containing a single nucleotide deletion in the 5'-noncoding region of the viral RNA. Virology. 1986 Dec;155(2):498–507. doi: 10.1016/0042-6822(86)90211-4. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Kremer M. J., Luo M., Vriend G., Arnold E., Kamer G., Rossmann M. G., McKinlay M. A., Diana G. D., Otto M. J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986 Sep 19;233(4770):1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Zeichhardt H., Otto M. J., McKinlay M. A., Willingmann P., Habermehl K. O. Inhibition of poliovirus uncoating by disoxaril (WIN 51711). Virology. 1987 Sep;160(1):281–285. doi: 10.1016/0042-6822(87)90075-4. [DOI] [PubMed] [Google Scholar]