Figure 1.
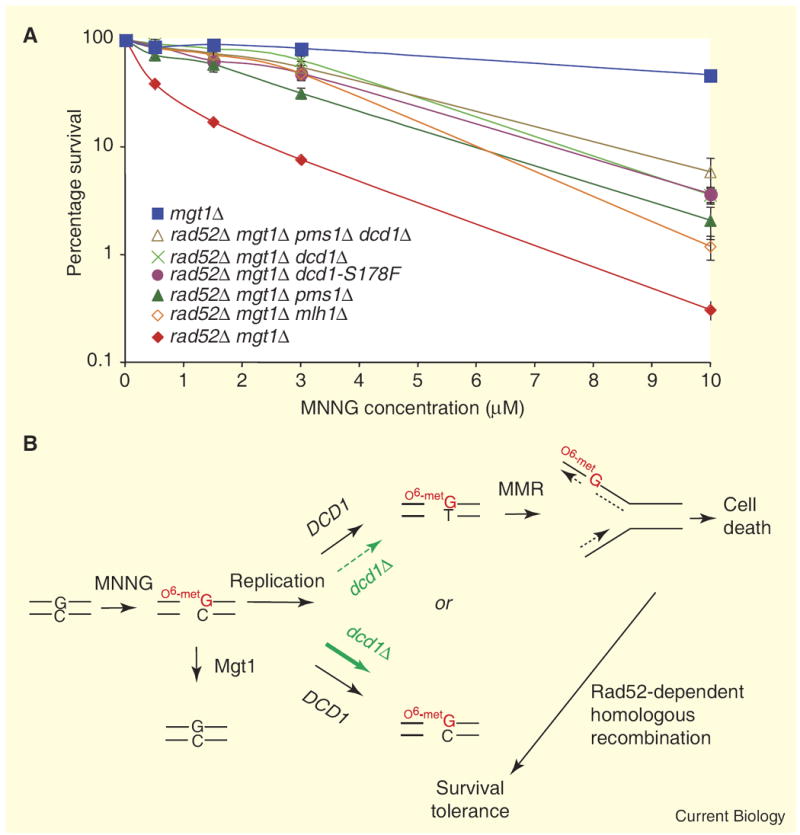
(A) MNNG survival curves: strains were exposed for 45 min to increasing amounts of MNNG. Error bars represent standard error of the mean. The LD50 values for mgt1Δ, rad52Δ mgt1Δ dcd1Δ pms1Δ, rad52Δ mgt1Δ dcd1Δ, rad52Δ mgt1Δ dcd1-S178F, rad52Δ mgt1Δ pms1Δ, rad52Δ mgt1Δ mlh1Δ and rad52Δ mgt1Δ strains were 9.7 μM, 2.4 μM, 2.2 μM, 2.1 μM, 1.8 μM, 1.6 μM and 0.25 μM MNNG, respectively. (B) A model for the cellular response to MNNG treatment in budding yeast. The scheme is modified from a previous report [2] to include the role of DCD1, which, when absent, results in reduced O6metG/T mispair formation following replication, reduced MMR-dependent processing and resistance to cell death. With the exception of the ‘heavy’ and ‘dashed’ arrows signifying consequences in the dcd1Δ strain, the model applies to wild-type yeast. Further, following replication, remaining O6metG/C residues will either be detoxified by the methyltransferase or diluted out by subsequent cell cycles. Although as shown the model infers MMR-dependent ‘futile repair’, ‘direct signaling’ must be considered (for reviews, see [1,12]). Support for the futile cycling model comes from several studies including in vitro results showing reiterative MMR excision cycles on O6metG/T mismatch-containing plasmid substrates [13]. Support for direct signaling comes from a ‘separation-of-function’ allele of mouse Msh6 that compromises spellchecking without affecting the DNA-damage response [14] and from studies showing that MutSα–MutLα complexes are required for ATR–ATRIP signaling from methylation damage sites [15].
