Abstract
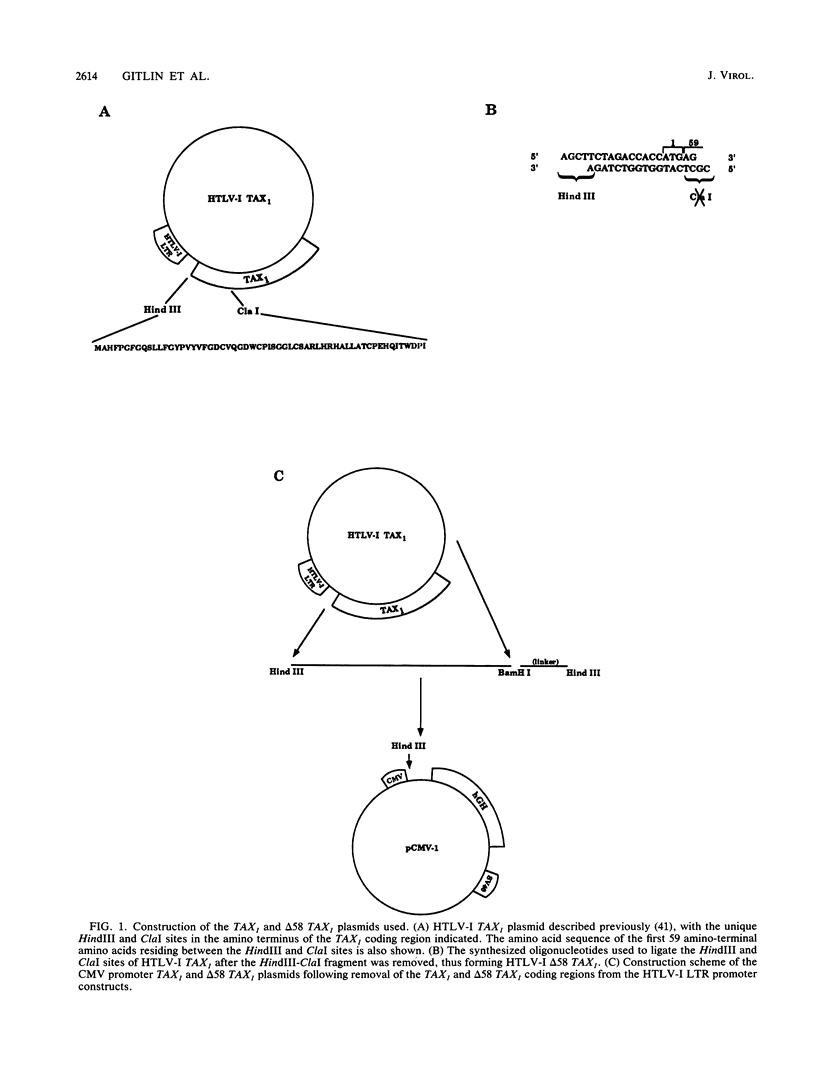
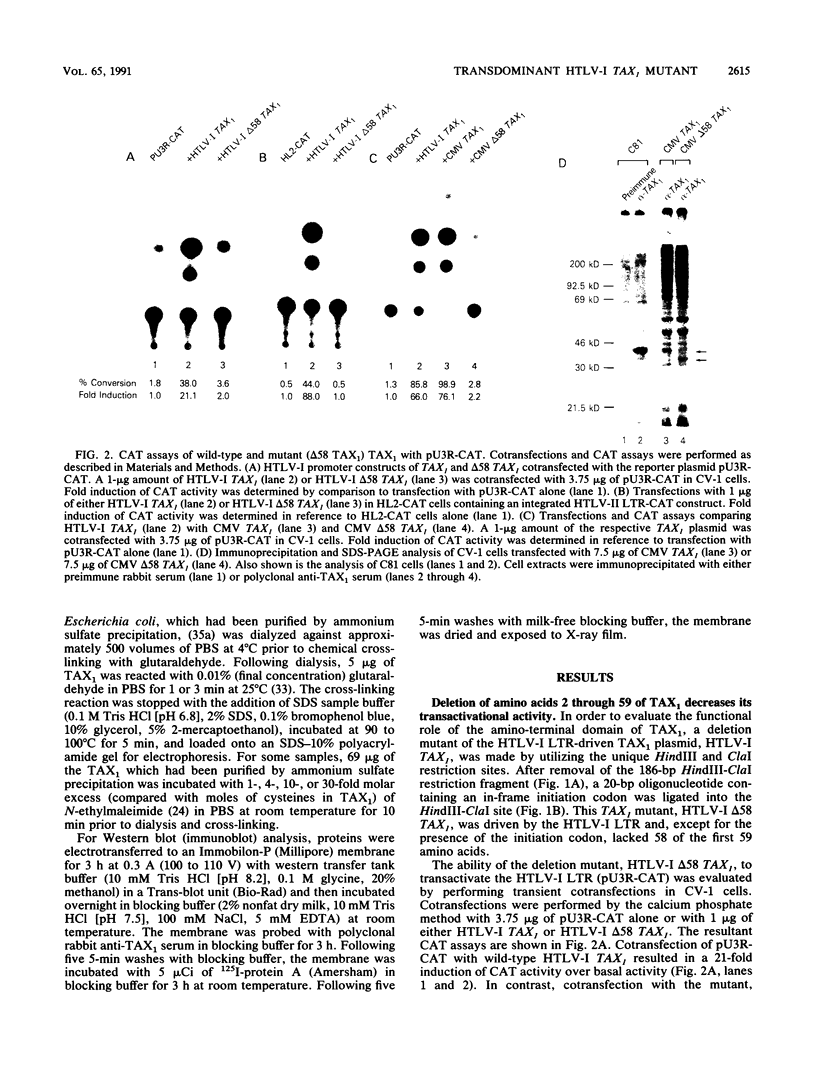
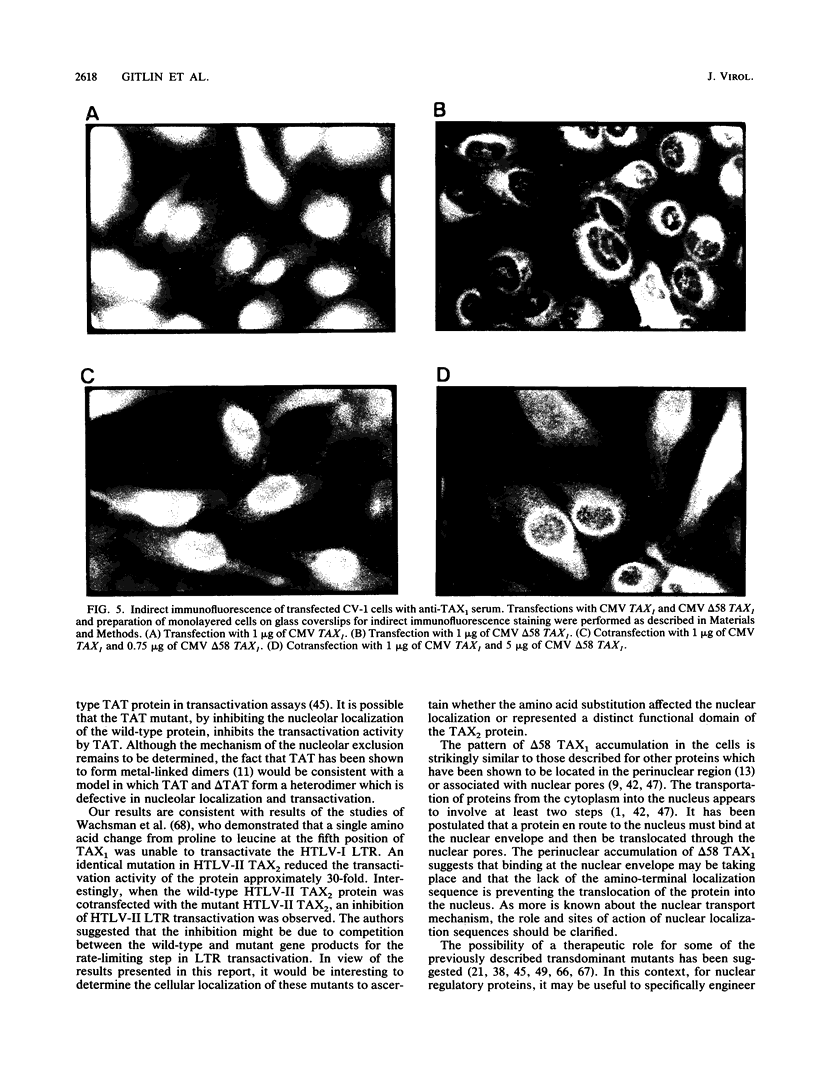
Human T-cell lymphotropic virus type I (HTLV-I) encodes a 40-kDa nuclear transactivating phosphoprotein, TAX1. The results presented in this study demonstrate that deletion of amino acids 2 through 59 of TAX1 (delta 58 TAX1) decreased transactivation of the HTLV-I long terminal repeat 10- to 20-fold. S1 nuclease analysis revealed that the decrease in transactivation of the HTLV-I long terminal repeat was associated with a lack of RNA synthesis. In contrast to the nuclear localization of the wild-type TAX1 protein, indirect immunofluorescence analysis demonstrated that delta 58 TAX1 failed to localize to the nucleus, indicating that the TAX1 nuclear localization sequence is present in amino acids 2 through 59. Cotransfection of wild-type and mutant TAX1 DNAs resulted in the cytoplasmic accumulation of TAX1 and a 25-fold decrease in transactivation. Although several possibilities which may account for this transdominant effect exist, we favor a model in which delta 58 TAX1 interferes with the nuclear localization of wild-type TAX1 protein, perhaps by forming heterodimer complexes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akey C. W., Goldfarb D. S. Protein import through the nuclear pore complex is a multistep process. J Cell Biol. 1989 Sep;109(3):971–982. doi: 10.1083/jcb.109.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Bangham C. R., Daenke S., Phillips R. E., Cruickshank J. K., Bell J. I. Enzymatic amplification of exogenous and endogenous retroviral sequences from DNA of patients with tropical spastic paraparesis. EMBO J. 1988 Dec 20;7(13):4179–4184. doi: 10.1002/j.1460-2075.1988.tb03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavati S., Ehrlich G., Kula R. W., Kwok S., Sninsky J., Udani V., Poiesz B. J. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988 May 5;318(18):1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Brady J., Jeang K. T., Duvall J., Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987 Jul;61(7):2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Szeberényi J., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990 Oct;10(10):5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988 Oct;8(10):4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Dworetzky S. I., Lanford R. E., Feldherr C. M. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol. 1988 Oct;107(4):1279–1287. doi: 10.1083/jcb.107.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Fujii M., Sassone-Corsi P., Verma I. M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharakhanian E., Takahashi J., Kasamatsu H. The carboxyl 35 amino acids of SV40 Vp3 are essential for its nuclear accumulation. Virology. 1987 Apr;157(2):440–448. doi: 10.1016/0042-6822(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Glenn G. M., Ricciardi R. P. An adenovirus type 5 E1A protein with a single amino acid substitution blocks wild-type E1A transactivation. Mol Cell Biol. 1987 Mar;7(3):1004–1011. doi: 10.1128/mcb.7.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. C., Sodroski J., Rosen C., Essex M., Haseltine W. A. Subcellular localization of the product of the long open reading frame of human T-cell leukemia virus type I. Science. 1985 Mar 8;227(4691):1227–1228. doi: 10.1126/science.2983419. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grassmann R., Dengler C., Müller-Fleckenstein I., Fleckenstein B., McGuire K., Dokhelar M. C., Sodroski J. G., Haseltine W. A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci U S A. 1989 May;86(9):3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. E., Hinrichs S. H., Vogel J., Jay G. Exocrinopathy resembling Sjögren's syndrome in HTLV-1 tax transgenic mice. Nature. 1989 Sep 7;341(6237):72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- Green M., Ishino M., Loewenstein P. M. Mutational analysis of HIV-1 Tat minimal domain peptides: identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell. 1989 Jul 14;58(1):215–223. doi: 10.1016/0092-8674(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Hanly S. M., Rimsky L. T., Malim M. H., Kim J. H., Hauber J., Duc Dodon M., Le S. Y., Maizel J. V., Cullen B. R., Greene W. C. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989 Oct;3(10):1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- Haugaard N., Cutler J., Ruggieri M. R. Use of N-ethylmaleimide to prevent interference by sulfhydryl reagents with the glucose oxidase assay for glucose. Anal Biochem. 1981 Sep 15;116(2):341–343. doi: 10.1016/0003-2697(81)90368-7. [DOI] [PubMed] [Google Scholar]
- Hidaka M., Inoue J., Yoshida M., Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988 Feb;7(2):519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T., Engleka K., Zhan X., Tokita Y., Forough R., Roeder D., Jackson A., Maier J. A., Hla T., Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990 Sep 28;249(4976):1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Raine C. S., Mingioli E. S., McFarlin D. E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988 Feb 11;331(6156):540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989 Mar 31;243(4899):1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Loeken M. R., Khoury G., Brady J. Stimulation of the adenovirus E2 promoter by simian virus 40 T antigen or E1A occurs by different mechanisms. Mol Cell Biol. 1986 Jun;6(6):2020–2026. doi: 10.1128/mcb.6.6.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Hauber J., Cullen B. R. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989 Jul 14;58(1):205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Yoshida M., Arai K. T-cell activation signals and human T-cell leukemia virus type I-encoded p40x protein activate the mouse granulocyte-macrophage colony-stimulating factor gene through a common DNA element. Mol Cell Biol. 1988 Dec;8(12):5581–5587. doi: 10.1128/mcb.8.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Langevin G. L., Singer R. H., Garcea R. L., Hereford L. M. Amino acid sequences that determine the nuclear localization of yeast histone 2B. Mol Cell Biol. 1987 Nov;7(11):4048–4057. doi: 10.1128/mcb.7.11.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerenberg M., Hinrichs S. H., Reynolds R. K., Khoury G., Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987 Sep 11;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Nosaka T., Siomi H., Adachi Y., Ishibashi M., Kubota S., Maki M., Hatanaka M. Nucleolar targeting signal of human T-cell leukemia virus type I rex-encoded protein is essential for cytoplasmic accumulation of unspliced viral mRNA. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9798–9802. doi: 10.1073/pnas.86.24.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L., Garcia J., Wu F., Modesti N., Nelson J., Gaynor R. A transdominant tat mutant that inhibits tat-induced gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5079–5083. doi: 10.1073/pnas.87.13.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzatti R., Vogel J., Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990 Jan;10(1):413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Rimsky L., Dodon M. D., Dixon E. P., Greene W. C. Trans-dominant inactivation of HTLV-I and HIV-1 gene expression by mutation of the HTLV-I Rex transactivator. Nature. 1989 Oct 5;341(6241):453–456. doi: 10.1038/341453a0. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. Location of cis-acting regulatory sequences in the human T-cell leukemia virus type I long terminal repeat. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6502–6506. doi: 10.1073/pnas.82.19.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S., Perkins A., Purcell R., Joung K., Sia R., Burghoff R., Haseltine W. A., Rosen C. A. Structural and functional characterization of human immunodeficiency virus tat protein. J Virol. 1989 Jan;63(1):1–8. doi: 10.1128/jvi.63.1.1-8.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S., Poteat H., Tan T. H., Kawakami K., Roeder R., Haseltine W., Rosen C. A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988 Jul 1;241(4861):89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Feinberg M. B., Holbrook N., Wong-Staal F., Greene W. C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A., Keegan L. P., Ptashne M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H., Shida H., Nam S. H., Nosaka T., Maki M., Hatanaka M. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell. 1988 Oct 21;55(2):197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Boyle W. J., Keith D. E., Press M. F., Golde D. W., Souza L. M. Subnuclear localization of the trans-activating protein of human T-cell leukemia virus type I. J Virol. 1988 Mar;62(3):680–686. doi: 10.1128/jvi.62.3.680-686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Press M. F., Souza L. M., Murdock D. C., Cline M. J., Golde D. W., Gasson J. C., Chen I. S. Studies of the putative transforming protein of the type I human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1427–1430. doi: 10.1126/science.2990027. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Szeberényi J., Cai H., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990 Oct;10(10):5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Takahashi C., Yamaoka S., Nosaka T., Maki M., Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Venkatesh L. K., Chinnadurai G. Mutants in a conserved region near the carboxy-terminus of HIV-1 Rev identify functionally important residues and exhibit a dominant negative phenotype. Virology. 1990 Sep;178(1):327–330. doi: 10.1016/0042-6822(90)90414-m. [DOI] [PubMed] [Google Scholar]
- Wachsman W., Cann A. J., Williams J. L., Slamon D. J., Souza L., Shah N. P., Chen I. S. HTLV x gene mutants exhibit novel transcriptional regulatory phenotypes. Science. 1987 Feb 6;235(4789):674–677. doi: 10.1126/science.3027894. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]








