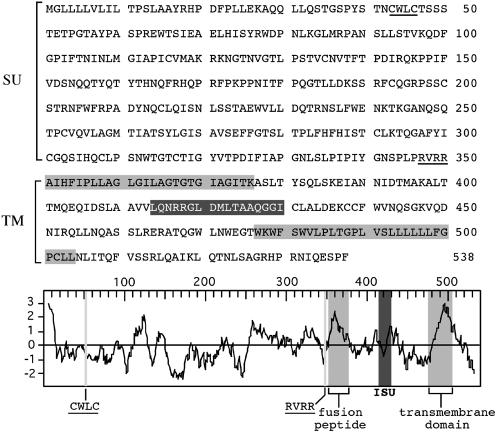
Fig. 2.
Primary sequence, hydrophobicity profile, and predicted features of the HERV-FRD envelope. The SU and TM moieties of the envelope are delineated, with the canonical RVRR cleavage site between the two subunits underlined (consensus: R/K-X-R/K-R); in the TM subunit, the hydrophobic fusion peptide and transmembrane domains are shaded in light gray, and the putative immunosuppressive domain (ISU) in dark gray; in the SU subunit, the canonical CWLC domain involved in SU-TM interaction is underlined.