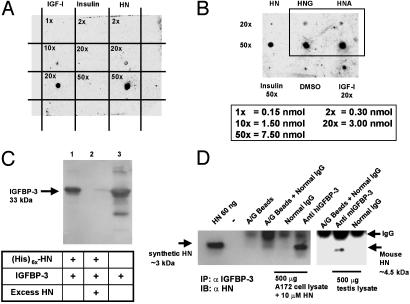
Fig. 1.
IGFBP-3 binds HN. (A) A ligand dot blot experiment showing IGFBP-3 binding to HN. HN, IGF-I, and insulin at 0.15–7.5 nmol were immobilized on a PVDF membrane and probed with125I-labeled IGFBP-3. (B) A ligand dot blot showing equal binding of immobilized HNG and HNA (at 3 and 7.5 nmol) to 125I-IGFBP-3. DMSO and insulin (7.5 nmol) are negative controls, and IGF-I (3 nmol) is a positive control. (C) Ni-NTA agarose column with immobilized (His)6x-HN elutes rhIGFBP-3. Western blot with anti-hIGFBP-3 is shown. Lanes: 1, (His)6x-HN (1 nmol) pull down of IGFBP-3 (1 nmol); 2, displacement of IGFBP-3 from (His)6x-HN with 100 molar excess of unlabeled HN; 3, rhIGFBP-3 positive control. (D) HN coimmunoprecipitates with IGFBP-3 (Left). As negative controls, eluates from beads containing protein A/G and normal goat IgG were used. (Right) Testes of 3-wk-old mice were homogenized in immunoprecipitation buffer and immunoprecipitated with either polyclonal goat anti-mouse-IGFBP-3 or normal goat IgG. Mouse HN runs at ≈4.5 kDa. Eluate from beads containing protein A/G plus IgG was used as negative control. Synthetic HN (60 ng of pure peptide) was used as positive control in both experiments. Results are representative of three independent experiments.