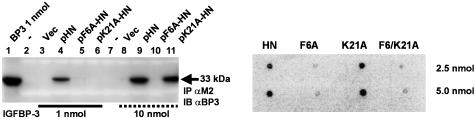
Fig. 2.
F6A-HN shows reduced IGFBP-3 binding. Alanine scanning of FLAG-tagged pHN, pF6A-HN, and p21A-HN revealed that F6A-HN has decreased affinity to IGFBP-3 when compared with K21A-HN. In contrast to 1 nmol IGFBP-3, at 10 nmol IGFBP-3, M2 antibody pulls down IGFBP-3 from pK21A-HN transfected cells, whereas in pF6A-HN transfected cells, IGFBP-3 does not coimmunoprecipitate. Vector alone was used as a negative control, and IGFBP-3 protein (1 nmol) was used as a positive control. Lysates were subjected to Western blotting with M2 antibody to ensure equal loading (data not shown) (Left). A ligand dot blot experiment confirmed that F6 is essential for IGFBP-3 binding (Right). F6A, K21A, and F6/K21A-HN at 2.5 or 5 nmol were immobilized on a PVDF membrane and probed with125I-labeled IGFBP-3. Both F6A and F6/K21A mutants showed significantly reduced binding. HN at 2.5 and 5 nmol was used as a positive control.