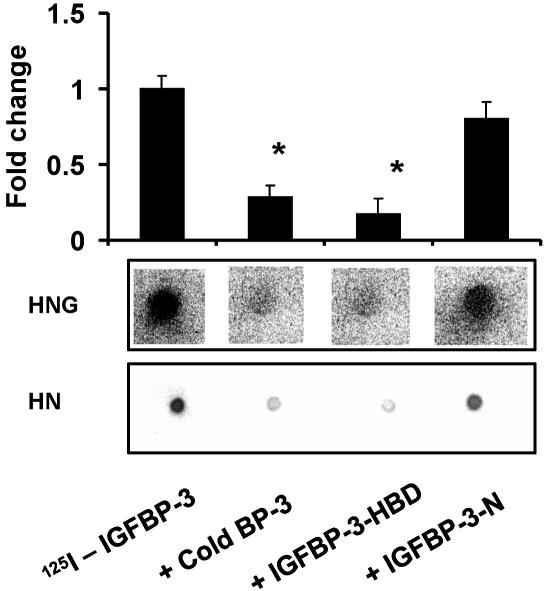
Fig. 3.
IGFBP-3 and HN binding is blocked by the HBD of IGFBP-3. HN/HNG–IGFBP-3 binding was assessed in a competitive ligand dot blot experiment, where HN (2.5 nmol) or HNG (7.5 nmol) immobilized onto PVDF membranes were probed with125I-rhIGFBP-3 either without competition or in the presence of 1 μM competing unlabeled125I-IGFBP-3, N-terminal 20-aa peptide of IGFBP-3 (IGFBP-3-N), or C-terminal 18-aa HBD peptide of IGFBP-3 (IGFBP-3-HBD). 125I signals were detected by using storm phosphorimager and imagequant software (Molecular Dynamics). HBD-IGFBP-3 competed to a similar extent as full-length rhIGFBP-3, whereas the IGFBP-3-N-terminal peptide did not block125IIGFBP-3–HN binding. The results are shown as a representative from three independent experiments for both HN and HNG. *, P < 0.05.