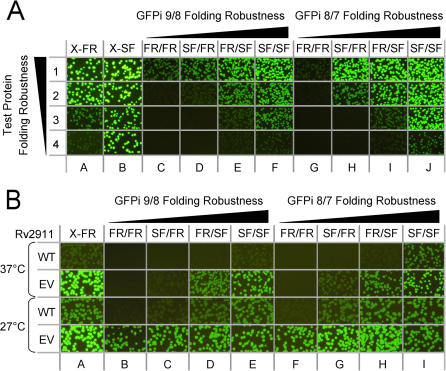
Figure 4. Sensitivity of various GFP insertion constructs (columns A through J) to protein misfolding.
(A) Images of E. coli cells on plates expressing four test fusions proteins with decreasing folding robustness at 37°C. Protein #1 sulfite reductase (dissimilatory subunit) is fully soluble (row 1); protein #2 (translation initiation factor) is 70% soluble (row 2), protein #3 (3-hexulose 6-phosphate synthase) is 50% soluble (row 3), and protein #4 (polysulfide reductase subunit) is fully insoluble (row 4). Test proteins expressed as fusions with C-terminal folding reporter (X-FR) (column A), superfolder (X-SF) GFP (column B), or inserted in the four GFPi 9/8 reporters (columns C–F), or GFPi 8/7 reporters (columns G–J). Designations above each column designate the GFP variant from which each flanking GFP domain is derived (see Fig. 3 ). Exposure time is 2 s. (B) Fluorescent images of E. coli colonies expressing GFP reporter constructs of wild-type insoluble Rv2911 from Mtb (rows marked WT) and its evolved variant engineered using the C-terminal folding reporter GFP (rows marked EV) at two temperatures (27°C and 37°C). Columns and their designations correspond to the same GFP topologies indicated in Fig. 4a (above). Fluorescence was imaged after IPTG induction at 37°C and 27°C. Exposure time is 2 s.