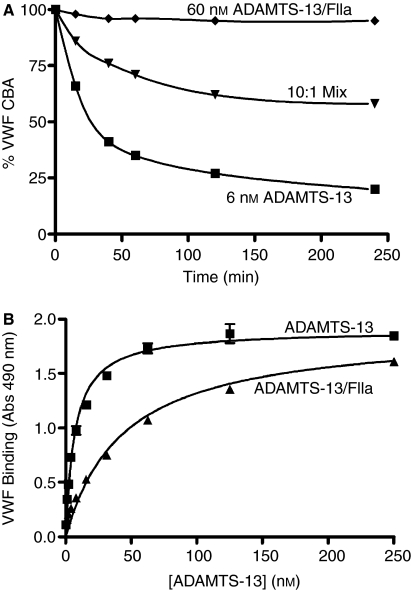
Fig. 3.
ADAMTS-13 proteolyzed by thrombin has reduced affinity for von Willebrand factor (VWF). (A) 6 nm ADAMTS-13, 60 nm thrombin-cleaved ADAMTS-13 (ADAMTS-13/FIIa) or 60 nm thrombin-cleaved ADAMTS-13/6 nm ADAMTS-13 (10:1 mix) was incubated with 10 nm purified plasma-derived VWF in the presence of 1.5 m urea and 5 mm BaCl2. At time-points (0–4 h) subsamples were stopped with EDTA and VWF function measured using a collagen-binding assay (CBA). Changes in CBA are represented as % original CBA at 0 h. A 10-fold molar excess of proteolyzed ADAMTS-13 partially competed with ADAMTS-13 for VWF. (B) The affinity of ADAMTS-13 and thrombin-cleaved ADAMTS-13 for purified plasma-derived VWF was measured by plate assay. 30 nm VWF was immobilized to microtiter wells, and incubated with varying concentrations of ADAMTS-13 or thrombin-cleaved ADAMTS-13 (ADAMTS-13/FIIa), as in Materials and methods. A high-affinity interaction between ADAMTS-13 and VWF was determined (KD∼6 nm), whereas this was appreciably reduced for ADAMTS-13/FIIa (KD∼45 nm).