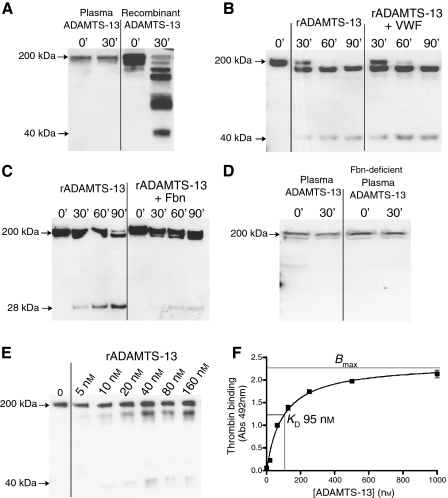
Fig. 5.
ADAMTS-13 is not efficiently proteolyzed by thrombin in plasma. (A) Endogenous ADAMTS-13 was detected in plasma by Western blotting using an anti-ADAMTS-13 polyclonal antibody (pAb). No change in the intensity of this band was observed 30 min after initiation of coagulation. Conversely, multiple cleavage products were generated when 100 nm recombinant ADAMTS-13 (rADAMTS-13) was incubated with 100 nm thrombin for 30 min and analyzed in the same way. (B) 50 nm rADAMTS-13 was incubated with 100 nm thrombin in the presence and absence of 500 nm von Willebrand factor. ADAMTS-13 fragmentation was analyzed from 0–90 min by Western blotting with an anti-ADAMTS-13 pAb. (C) 100 nm rADAMTS-13 was incubated with thrombin in the presence and absence of 7 μm human fibrinogen (Fbn). ADAMTS-13 fragmentation was analyzed from 0–90 min by Western blotting with an antimetalloprotease domain monoclonal antibody. (D) Endogenous plasma ADAMTS-13 was detected in normal plasma and plasma from afibrinogenemic patients (Fbn-deficient) as in (A). No change in the intensity of the ADAMTS-13 band was observed 30 min after initiation of coagulation in either plasma. (E) 5–160 nm rADAMTS-13 was incubated with 25 nm thrombin for 60 min and thereafter ADAMTS-13 fragmentation was analyzed as in (A). ADAMTS-13 prior to thrombin incubation (0′) is shown. For each concentration, the same amount of ADAMTS-13 was used for Western blotting. (F) The affinity of ADAMTS-13 for thrombin was measured by plate assay. 80 nm active site blocked thrombin was immobilized to microtiter wells, and incubated with varying concentrations of rADAMTS-13, as in Materials and methods. A KD∼95 nm was determined.