Scheme 4.
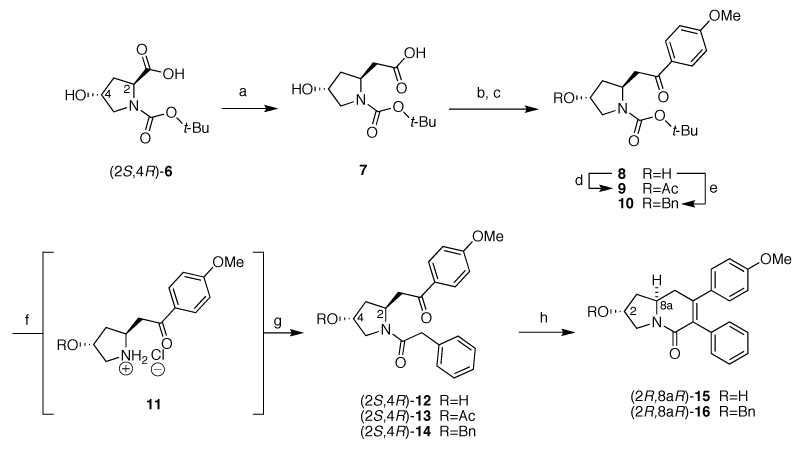
Synthesis of tetrahydroindolizin-5(1H)-one intermediates (R,R)-15 and (R,R)-16. Reagents and conditions: a) i) TEA, ClCO2Et, diazomethane, THF, 0 °C-RT, 12 h; ii) PhCO2Ag, TEA, −25 °C-RT, 12h, (27%, unoptimized); b) N,O-dimethylhydroxylammonium chloride, EDCI, NMM, dry CH2Cl2, 0 °C-RT, 2h (98%); c) Mg0 turnings, cat. I2 crystals, 4-bromoanisol, dry THF, (85%); d) Ac2O, DMAP, pyridine, 0 °C-RT, 1h (99%); e) Ag2O, BnBr, toluene, 50 °C, 3h (74%); f) 4 M HCl/dioxane, (for R=H a 77:23 dr was noted; for R=Ac, Bn the dr was not determined); g) phenylacetic acid, EDCI, NMM, dry CH2Cl2, (R=H 38%, 74:26 dr), (R=Ac 88%, 80:20 dr), (R=Bn, 73%, 60:40 dr); h) KOHMeOH, reflux (see Table 2).
