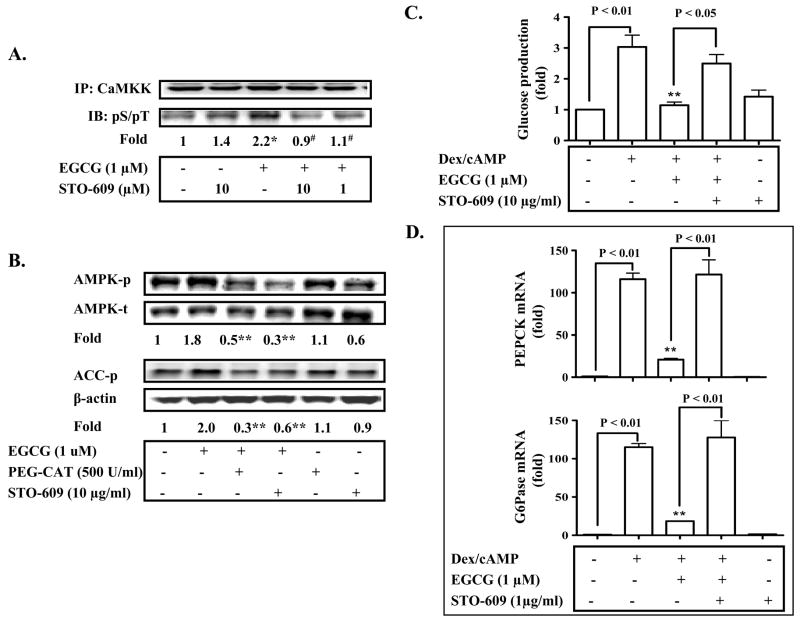
Fig. 5. EGCG activates AMPK through Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) and ROS, and consequently inhibits hepatic gluconeogenesis.
(A) Primary hepatocytes were treated with EGCG for 10 min. CaMKK molecules in the cells were then immunoprecipitated (IP) with the specific antisera against CaMKK, and presence of phosphorylated CaMKK was detected by immunoblotting (IB) with antibodies against phosphorylated serine/threonine. Levels of phospho-CaMKK were normalized to the total CAMKK. *: P < 0.05 compared to all other lanes; #: P < 0.05 compared to EGCG treatment. (B) Primary hepatocytes were pre-treated with a CaMKK inhibitor, STO-609, or a cell membrane permeable catalase (PEG-CAT) for 30 min prior to the treatment with EGCG (10 min). Levels of phospho-/total AMPK, phospho-ACC, and β-actin were measured by immunoblotting, and quantified by densitometry. **: p < 0.01 vs. EGCG alone. (C) and (D) Hepatocytes were pre-incubated with STO-609 for 30 min as noted, incubated with EGCG or insulin for 3 h in the serum-free Williams’ E medium, and were then washed with the pre-warmed glucose-free DMEM medium 3 times. Cells were subsequently treated with cAMP/dexamethasome (Dex) in the presence of EGCG or insulin for 3 h in the glucose-free DMEM medium. Gluconeogenic substrates were added to some cells. The glucose production via gluconeogenesis was quantified and calculated as detailed in “Materials and Methods”. Transcripts of PEPCK and G6Pase genes were quantified by the Taqman Real-time PCR and normalized to GADPH. **: P < 0.01 vs. cells treated with Dex/cAMP.