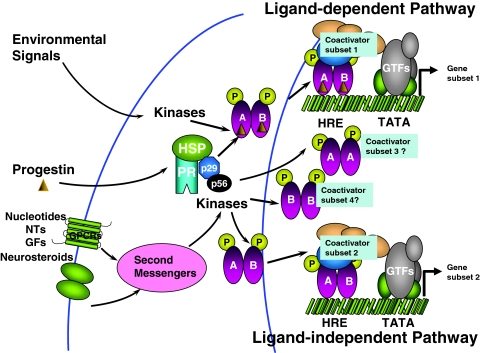
Figure 2.
Schematic representation of integrated PR activation. Unliganded PR is present as an inactive complex associated with heat-shock proteins (HSP) and chaperone proteins (p29, p56). In the classical ligand-dependent pathway, P and other progestins bind to the PR, resulting in conformational change, dissociation of HSP and chaperone proteins, dimerization of the receptor, and binding to the hormone response element (HRE) in the target DNA. The P-induced conformational change facilitates the recruitment of cofactors and other general transcription factors (GTFs) to the promoter, producing a transcriptionally active complex that can direct gene transcription. Compounds such as cyclic nucleotides, neurotransmitters (NTs), growth factors (GFs), neurosteroids, and other environmental signals can activate second messengers and protein kinase pathways to activate PR and/or coactivators in a ligand-independent manner. Depending upon the activating stimulus, PRs can dimerize as homodimers (A:A, B:B) or heterodimers (A:B) and upon binding to HRE can recruit distinct sets of coactivators, leading to transcriptional activation of distinct subsets of genes.