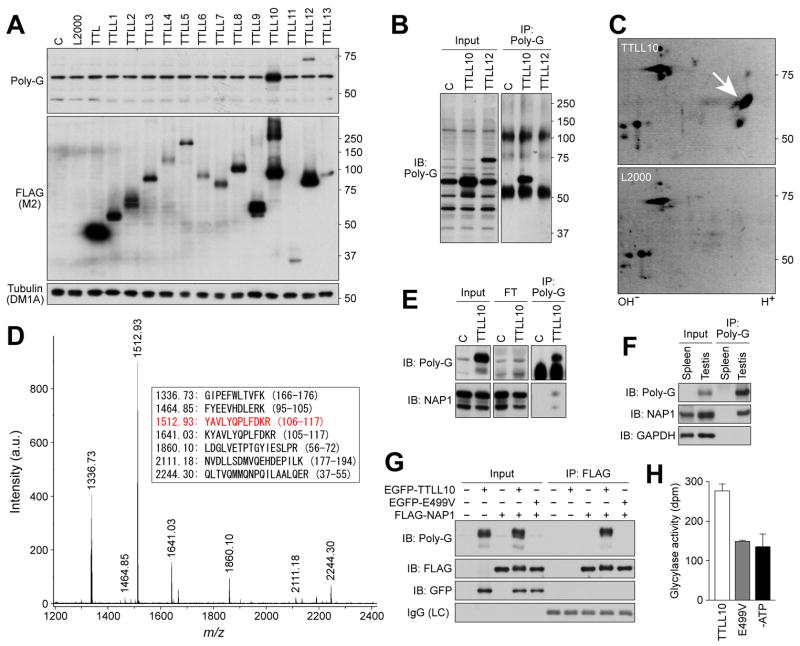
Figure 2. Identification of TTLL10 as a glycine ligase for NAP1 in vitro and in vivo.
(A) Screening of HEK293T cells expressing thirteen TTLL proteins with the R-polygly antibody. In TTLL10-expressing cell lysates, there was a significant increase in a 60-kDa protein detected by the antibody. (B) Immunoprecipitation of the 60-kDa protein. Note that non-specific R-polygly reactive proteins present in control samples and TTLL12-generated proteins were not precipitated with the antibody. (C) Two-dimensional gel electrophoresis resolution of the immunoprecipitated protein. Arrow indicates spots observed only in TTLL10 expressing sample. (D) Tandem mass spectrometry of trypsin digests of the immunoprecipitated protein. The sequences corresponding to the peaks are listed in the box. The peptide sequence in red was further identified by tandem mass spectrometry. (E-G) Immunoprecipitation-western blot analyses. Protein immunoprecipitated from TTLL10-expressing cell lysates or testis lysates with the R-polygly antibody was detected by an anti-NAP1 antibody (E,F). Almost no glycylated proteins remained in flow through (FT) in panel E. FLAG-NAP1 immunoprecipitated from lysates of cells co-expressing TTLL10 was detected by the R-polygly antibody (G). Note that FLAG-NAP1 co-expressed with wild-type TTLL10 displayed both slightly slower mobility and blurred tailing in electrophoresis, indicative of change of molecular weight by addition of multiple glycines (G). (H) In vitro enzyme activity of TTLL10. The activity was measured by [3H]-glycine incorporation into recombinant NAP1.