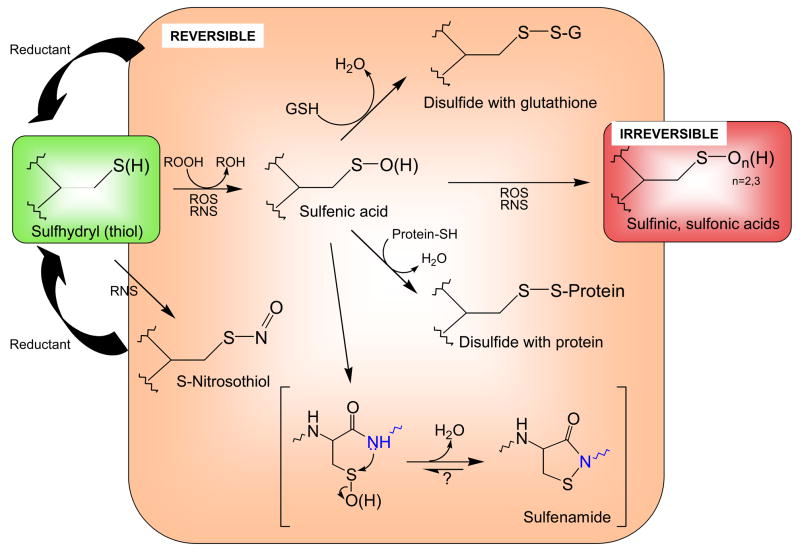
Figure 1. Biological modifications of cysteine thiols.
Reactive cysteine thiols (green), typically in their ionized, thiolate form (R-S−), are oxidized by such oxidants as hydrogen peroxide, organic hydroperoxides, hypochlorous acid and peroxynitrite to form sulfenic acids, which may be stabilized or go on to form other reversible (disulfides or sulfenamides, orange) or irreversible (sufinic and sulfonic acid, red) species. Both reactive oxygen species (ROS) and reactive nitrogen species (RNS) promote these oxidations. Note that the generation of sulfenamide (bottom) involves attack of a neighboring amino acid’s amide nitrogen (blue) on the sulfenic acid sulfur. Although sulfinic and sulfonic acids are shown here as irreversible modifications, recent discoveries show that some peroxiredoxins in this state can be recovered through action of specialized sulfinic acid reductases (sulfiredoxins).