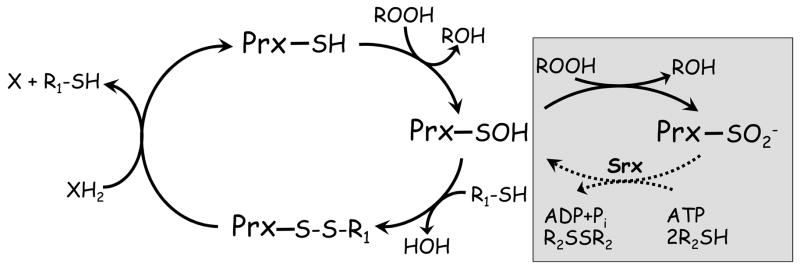
Figure 2. Peroxiredoxin catalytic and regulatory redox cycles.
Peroxiredoxins (Prx) have in common the first step of catalysis whereby the active site cysteine thiol (in its thiolate form) attacks the hydroperoxide substrate, releasing the corresponding alcohol and the enzyme in its sulfenic acid (SOH) form. For catalytic recycling, a resolving cysteine (R1-SH) in the same or another subunit typically forms a disulfide bond with the peroxidatic cysteine, and the enzyme is regenerated by small molecule or protein electron donors. The sulfenic acid can also act as a redox-sensitive switch, converting the enzyme to an inactive sulfinic acid form in the presence of excess hydroperoxide substrate (gray box). Enzymes called sulfiredoxins (and perhaps sestrins) can regenerate activity in some Prx through an ATP-dependent reduction (dotted line).