Abstract
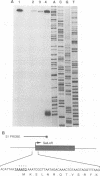
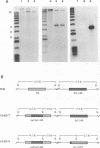
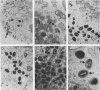
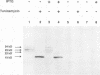
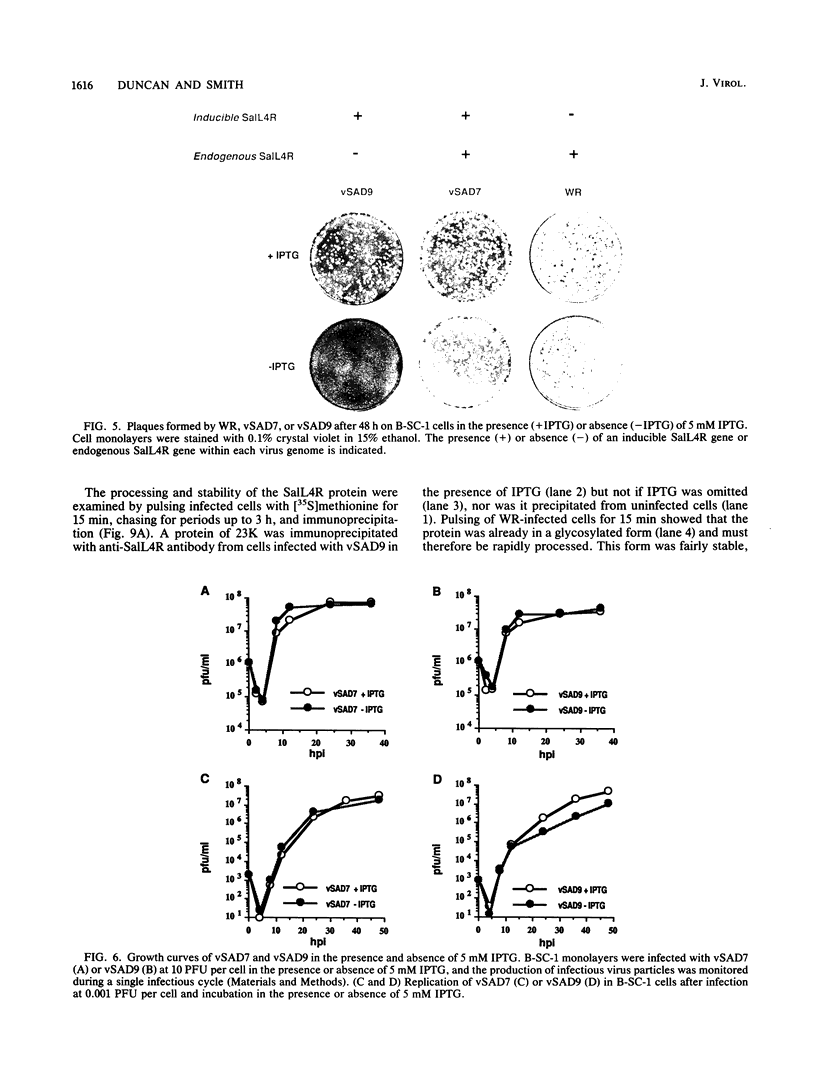
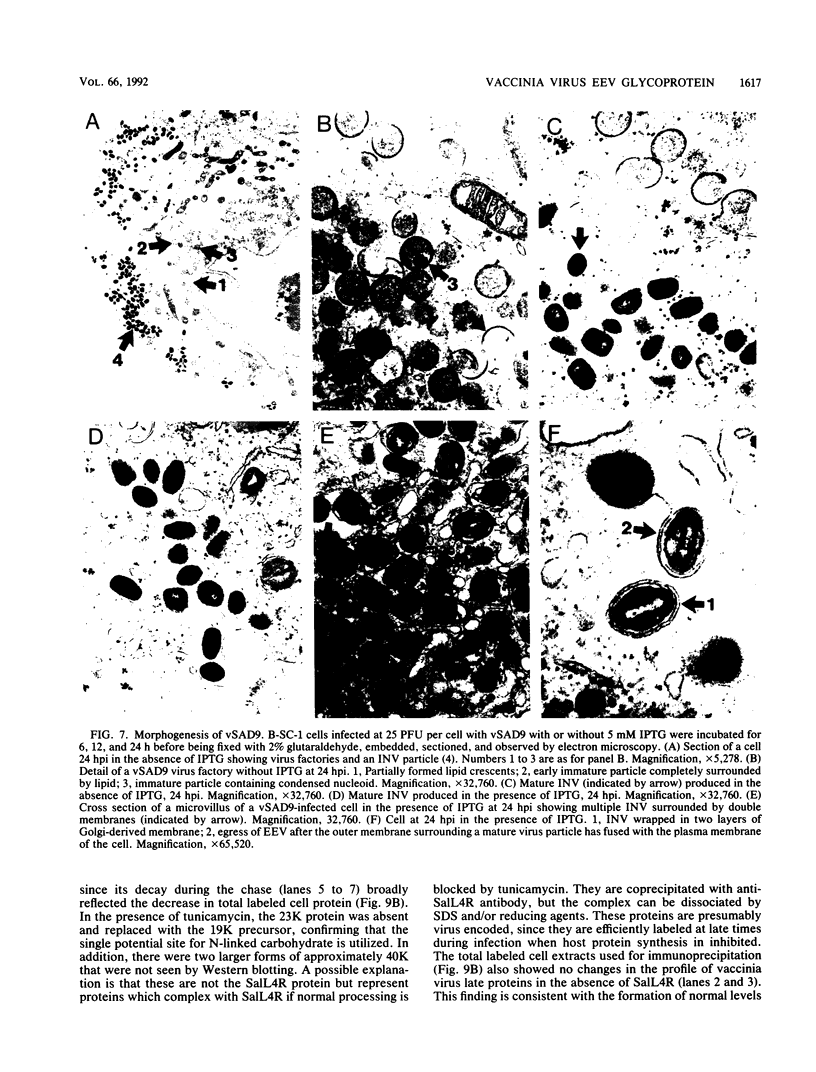
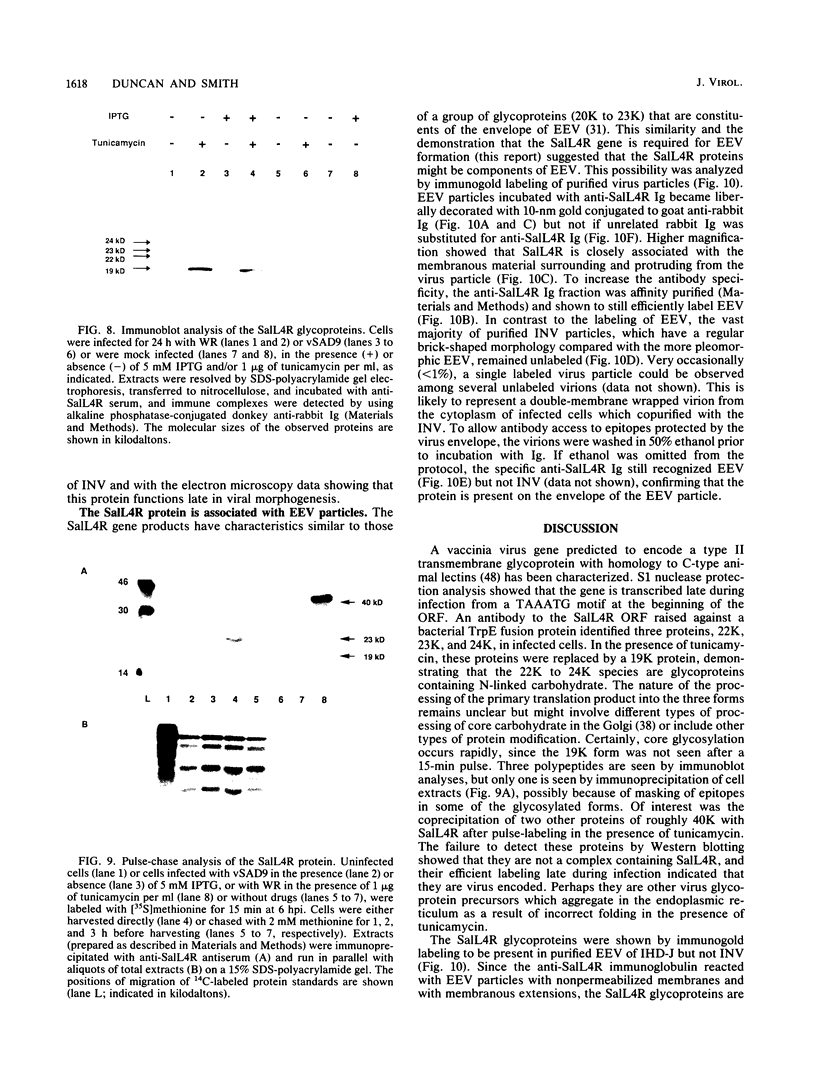
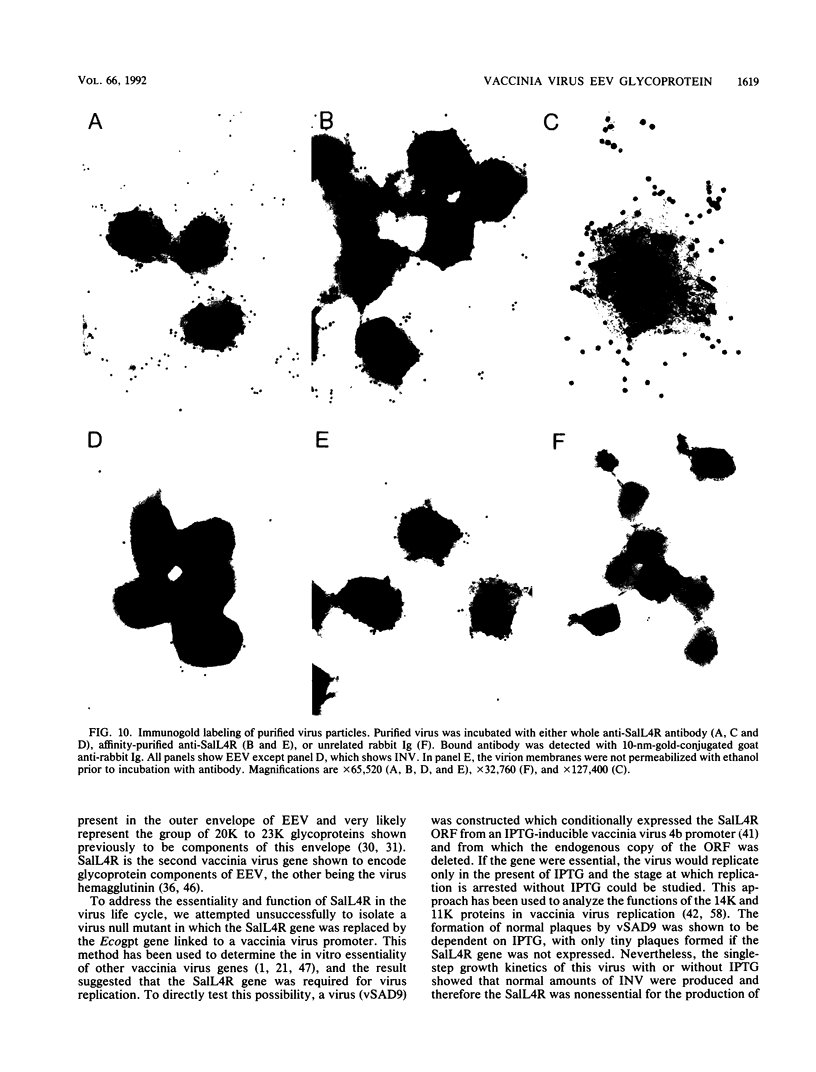
Sequence analysis of the vaccinia virus strain Western Reserve genome revealed the presence of an open reading frame (ORF), SalL4R, which has the potential to encode a transmembrane glycoprotein with homology to C-type animal lectins (G. L. Smith, Y. S. Chan, and S. T. Howard, J. Gen. Virol. 72:1349-1376, 1991). Here we show that the SalL4R gene is transcribed late during infection from a TAAATG motif at the beginning of the ORF. Antisera raised against a TrpE-SalL4R fusion protein identified three glycoprotein species of Mr 22,000 to 24,000 in infected cells. Immunogold electron microscopy demonstrated that SalL4R protein is present in purified extracellular enveloped virus particles but not in intracellular naked virus (INV). A mutant virus was constructed by placing a copy of the SalL4R ORF downstream of an isopropyl-beta-D-thiogalactopyranoside (IPTG)-inducible vaccinia virus promoter within the thymidine kinase locus and subsequently deleting the endogenous SalL4R gene. The growth kinetics of this virus demonstrated that SalL4R was nonessential for the production of infectious INV but was required for virus dissemination. Consistent with this finding, the formation of wild-type-size plaques by this mutant was dependent on the presence of IPTG. Electron microscopy showed that without SalL4R expression, the inability of the virus to spread is due to a lack of envelopment of INV virions by Golgi-derived membrane, a morphogenic event required for virus egress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991 Jul;183(1):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Boulter E. A., Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988 May 15;65(1):123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988 Mar;62(3):866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Palumbo G. J. Poxvirus pathogenesis. Microbiol Rev. 1991 Mar;55(1):80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Blumenthal R., Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990 Oct;64(10):4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
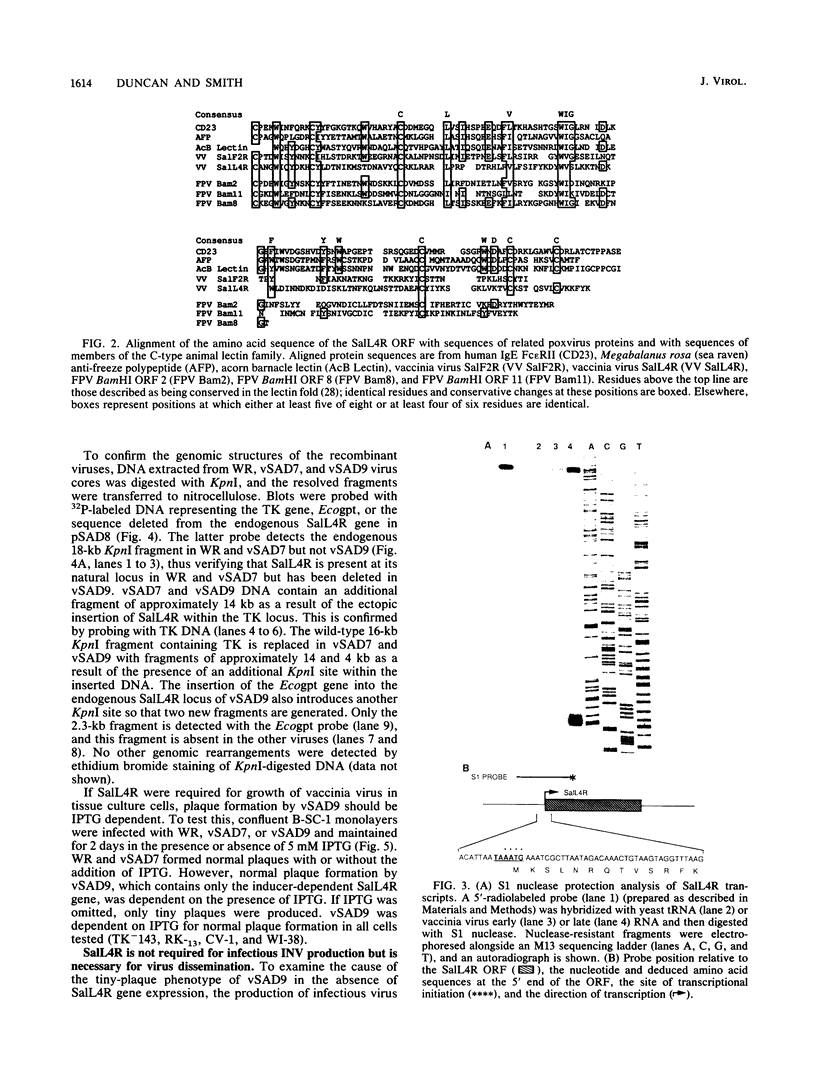
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Evans E., Traktman P. Molecular genetic analysis of a vaccinia virus gene with an essential role in DNA replication. J Virol. 1987 Oct;61(10):3152–3162. doi: 10.1128/jvi.61.10.3152-3162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Eibl H., Weber K. Characterization of intracellular and extracellular vaccinia virus variants: N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J Virol. 1981 Sep;39(3):903–913. doi: 10.1128/jvi.39.3.903-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt P., Hiller G., Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986 Jun;58(3):757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi M., Bannwarth W., Stunnenberg H. G. Conserved TAAAT motif in vaccinia virus late promoters: overlapping TATA box and site of transcription initiation. EMBO J. 1986 May;5(5):1071–1076. doi: 10.1002/j.1460-2075.1986.tb04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Kerr S. M., Smith G. L. Vaccinia virus DNA ligase is nonessential for virus replication: recovery of plasmids from virus-infected cells. Virology. 1991 Feb;180(2):625–632. doi: 10.1016/0042-6822(91)90076-n. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. On the predictive recognition of signal peptide sequences. Virus Res. 1985 Oct;3(3):271–286. doi: 10.1016/0168-1702(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of cytotoxic protein of eosinophilic leukocytes with IgE receptor Fc epsilon RII: implications for its structure and function. Mol Immunol. 1989 Dec;26(12):1151–1154. doi: 10.1016/0161-5890(89)90059-x. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Sadler J. E., Hill R. L. Restoration of specific myxovirus receptors to asialoerythrocytes by incorporation of sialic acid with pure sialyltransferases. J Biol Chem. 1979 Mar 25;254(6):2120–2124. [PubMed] [Google Scholar]
- Payne L. G. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J Virol. 1979 Jul;31(1):147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristensson K. Effect of glycosylation inhibitors on the release of enveloped vaccinia virus. J Virol. 1982 Feb;41(2):367–375. doi: 10.1128/jvi.41.2.367-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristensson K. The effect of cytochalasin D and monensin on enveloped vaccinia virus release. Arch Virol. 1982;74(1):11–20. doi: 10.1007/BF01320778. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Payne L. G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980 Sep;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Janeczko R., Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985 Nov;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Paez E., Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987 Feb;61(2):395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990 Sep 25;18(18):5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. Inducible gene expression from vaccinia virus vectors. Virology. 1990 Jul;177(1):239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz C., Payne L. G., Gubser J., Wittek R. A mutation in the gene encoding the vaccinia virus 37,000-M(r) protein confers resistance to an inhibitor of virus envelopment and release. J Virol. 1991 Jul;65(7):3435–3442. doi: 10.1128/jvi.65.7.3435-3442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Shida H. Nucleotide sequence of the vaccinia virus hemagglutinin gene. Virology. 1986 Apr 30;150(2):451–462. doi: 10.1016/0042-6822(86)90309-0. [DOI] [PubMed] [Google Scholar]
- Shuman S., Golder M., Moss B. Insertional mutagenesis of the vaccinia virus gene encoding a type I DNA topoisomerase: evidence that the gene is essential for virus growth. Virology. 1989 May;170(1):302–306. doi: 10.1016/0042-6822(89)90384-x. [DOI] [PubMed] [Google Scholar]
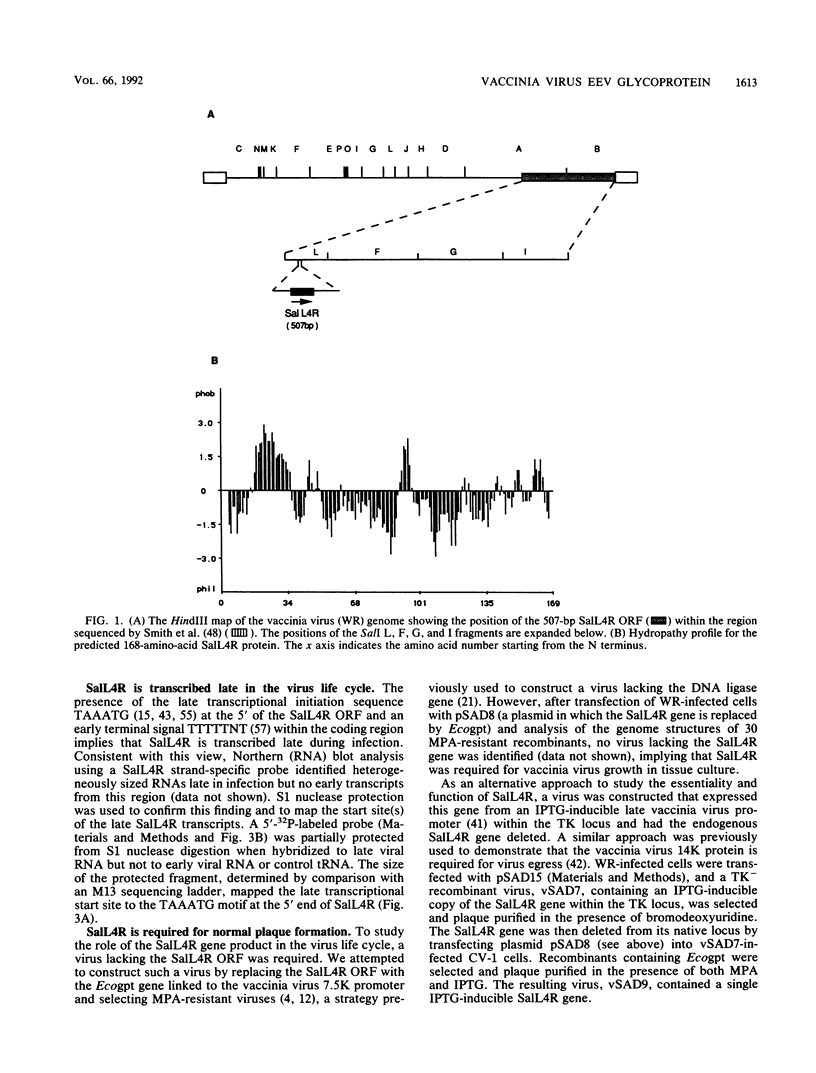
- Smith G. L., Chan Y. S., Howard S. T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991 Jun;72(Pt 6):1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Kerr S. M. Transcriptional mapping and nucleotide sequence of a vaccinia virus gene encoding a polypeptide with extensive homology to DNA ligases. Nucleic Acids Res. 1989 Nov 25;17(22):9051–9062. doi: 10.1093/nar/17.22.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Taylor J., Paoletti E. Fowlpox virus as a vector in non-avian species. Vaccine. 1988 Dec;6(6):466–468. doi: 10.1016/0264-410x(88)90091-6. [DOI] [PubMed] [Google Scholar]
- Tomley F., Binns M., Campbell J., Boursnell M. Sequence analysis of an 11.2 kilobase, near-terminal, BamHI fragment of fowlpox virus. J Gen Virol. 1988 May;69(Pt 5):1025–1040. doi: 10.1099/0022-1317-69-5-1025. [DOI] [PubMed] [Google Scholar]
- Traktman P. Poxviruses: an emerging portrait of biological strategy. Cell. 1990 Aug 24;62(4):621–626. doi: 10.1016/0092-8674(90)90106-o. [DOI] [PubMed] [Google Scholar]
- Weinrich S. L., Hruby D. E. A tandemly-oriented late gene cluster within the vaccinia virus genome. Nucleic Acids Res. 1986 Apr 11;14(7):3003–3016. doi: 10.1093/nar/14.7.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S., Stern W., Dales S. Biogenesis of vaccinia. Effects of inhibitors of glycosylation on virus-mediated activities. Virology. 1977 May 1;78(1):315–322. doi: 10.1016/0042-6822(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]