Abstract
The aim of the study was to evaluate the distribution of apoptosis in the medullary nuclei of infants and adults who died of hypoxic-ischaemic injury. Apoptosis was studied by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) in brainstems from 22 adults (7 subjects who died of opiate intoxication, 15 who died of other hypoxic-ischaemic injury) and 10 infants. The nuclei examined included the hypoglossal, dorsal motor nucleus of the vagus, nucleus tractus solitarii, nucleus of the spinal trigeminal tract, cuneate, vestibular and inferior olivary nuclei. A morphometric analysis with the optical disector method was performed to calculate the mean percentages (± standard deviation) of TUNEL-positive neuronal and glial cells for the sample populations. Opiate deaths did not have higher apoptotic indices than other adult hypoxic-ischaemic deaths. Statistically significant differences between adults and infants were found in the neuronal apoptotic indices of the cuneate (28.2 ± 16.3% vs. 6.9 ± 8.7%), vestibular (24.7 ± 15.0% vs. 11.3 ± 11.4%), nucleus tractus solitarii (11.2 ± 11.2% vs. 2.3 ± 2.4%), dorsal motor nucleus of the vagus (6.8 ± 8.5% vs. 0.1 ± 0.2%) and hypoglossal (6.6 ± 5.7% vs. 0.1 ± 0.2%), indicating higher resistance of the neuronal populations of these infant medullary nuclei to terminal hypoxic-ischaemic injury or post-mortem changes. Differences in neuronal apoptotic index were also statistically significant among nuclei, suggesting differential characteristics of survival. Nuclei with higher neuronal apoptotic indices were the cuneate, vestibular and nucleus of the spinal trigeminal tract, which are located in the lateral medullary tegmentum and share the same vascular supply from the posterior inferior cerebellar artery.
Keywords: apoptosis, brainstem, human, medulla oblongata, neuropathology, opiates, TUNEL
Introduction
In the literature, quite specific anatomic patterns of necrosis have been reported in the medulla oblongata of neonates with perinatal hypoxia (Gilles, 1969; Dambska et al. 1976; Leech & Alvord, 1977; Janzer & Friede, 1980; Taylor & Roessman, 1984; Pindur et al. 1992) and of adults with hypoxic-ischaemic injury (Gilles, 1969; Revesz & Geddes, 1988; De Caro et al. 2000, 2003; Porzionato et al. 2004a; Parenti et al. 2005). Moreover, earlier studies have found specific patterns of vascularization in the medullary nuclei, showing different microvessel densities (Porzionato et al. 2004b, 2005). Neurons may also undergo apoptosis in response to hypoxic-ischaemic injury (Snider et al. 1999). Apoptosis is a common type of programmed cell death, characterized by chromatin condensation and margination, cell shrinkage, membrane blebbing, and release of apoptotic bodies (Kerr et al. 1972). It involves the activation of caspases, which cleave DNA molecules into small fragments and disintegrate the cell into apoptotic bodies. The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) is a method which can identify such DNA strand breaks (Wyllie, 1980). In the central nervous system, apoptosis has mainly been studied with regard to embryonic development (Chan & Yew, 1998), ageing (Anglade et al. 1997) and cerebrovascular diseases (Zhang et al. 2004), with particular reference to diencephalic and telencephalic structures; literature data regarding the topographic distribution of apoptotic phenomena in human infant and adult medulla oblongata are still lacking. The present study was designed to evaluate the distribution of apoptosis of neurons and glial cells in the medullary nuclei of infant and adult subjects who died of hypoxic-ischaemic injury, to identify possible anatomic and age-related patterns.
Although many studies have reported the apoptotic effect of opiates in animals and cell cultures (Yin et al. 1997; Goswami et al. 1998; Hu et al. 2002; Atici et al. 2004), apoptosis in human heroin addicts has not yet been studied. Thus, a comparison was also made between opiate addicts who died of heroin intoxication and the other adult cases, taken as a control group.
Materials and methods
Materials
The present study was performed on 32 medullae oblongatae sampled during autopsy from the following subjects: 15 adults (9 male, 6 female; age range: 25–58 years; mean age: 40 years; mean death–autopsy interval (DAI) ± SD: 29 ± 2.1 h) who died of myocardial infarct (10 cases), haemorrhagic shock (3 cases) or bronchopneumonia (2 cases); 7 adults (7 male; age range: 20–38 years; mean age: 30 years; mean DAI ± SD: 31 ± 1.9 h) who died of opiate intoxication; 10 infants (5 male, 5 female; age range: 1 month to 1 year; mean age: 5 months; mean DAI ± SD: 30 ± 2.0 h) who died of sudden infant death syndrome (SIDS) (5 cases), bronchopneumonia (3 cases) or cardiopathologic causes (2 cases).
As regards opiate deaths, confirmation of heroin intoxication and a search for other drugs of abuse (cocaine, methadone, amphetamine, benzodiazepines, cannabis, alcohol) were performed by toxicological immunochemical screening (Enzyme Multiplied Immunoassay Technique) and confirmatory chromatographic techniques (High-Performance Liquid-Chromatography and Gas-Chromatography, coupled with Mass Spectrometry in Selective Ion Monitoring mode), on urine and venous blood samples.
Histological and TUNEL techniques
Autopsies were performed within 36 h of death. In all cases, macroscopic and microscopic examination revealed the absence of acute, chronic, localized or diffuse brain pathology. Brainstems were fixed in 10% formalin for 7 days and the medullae oblongatae were cut into 10-µm-thick transverse serial sections. In each case, five sections, collected 100 µm from each other at the level of the caudal portion of the IV ventricle, were examined by the TUNEL method. Adjacent sections were stained with haematoxylin-eosin, Nissl, Klüver-Barrera and azan-Mallory.
Sections were incubated with 20 µg mL−1 proteinase K for 15 min at room temperature and washed in 0.01 m phosphate-buffered saline (PBS). They were incubated in 0.3% hydrogen peroxide in deionized H2O to arrest endogenous peroxidase activity, and then incubated with equilibration buffer (ApopTag, Oncor, Gaithersburg, MD, USA) for 5 min at room temperature. Sections were treated with terminal-deoxynucleotidyl transferase reaction mix for 1 h at room temperature, soaked in stop-wash buffer, and incubated for 15 min at room temperature. Sections were then incubated with horseradish peroxidase streptoavidin, diluted 1 : 500 in PBS, for 30 min at room temperature. After the reaction, sections were washed with PBS, placed in 0.03% 3,3′-diaminobenzidine (DAB, Sigma, Milan, Italy) containing 0.01% H2O2, and counterstained with haematoxylin. For positive controls, incubation with DNase was performed. For negative controls, the terminal-deoxynucleotidyl transferase reaction mix was omitted.
Morphometric analysis
Sections were examined under a Leica DM4500B microscope (Leica Microsystems, Wetzlar, Germany) with a Leica DFC320 high-resolution digital camera (Leica Microsystems), and image data were transmitted to a personal computer equipped with appropriate software for image acquisition and analysis (qwin, Leica Microsystems). The nuclei examined included the hypoglossal (XII), dorsal motor nucleus of the vagus (DMNV), nucleus tractus solitarii (NTS), medial vestibular nucleus (Ve), cuneate nucleus (Cu), nucleus of the spinal trigeminal tract (NSTT), principal inferior olivary nucleus (PION), medial inferior olivary nucleus (MION) and dorsal inferior olivary nucleus (DION) (Fig. 1). The boundaries of these nuclei were defined according to McRitchie & Tork (1993) and Paxinos & Huang (1995). In each section, they were traced as regions of interest. Haematoxylin counterstaining in sections stained with the TUNEL method usually permitted precise delineation of the boundaries of the medullary nuclei. Moreover, tracing of nuclear boundaries was also confirmed by comparison with the adjacent serial sections, stained with haematoxylin-eosin, Nissl, Klüver-Barrera and azan-Mallory. For DMNV, dorsal and medial fringes were not considered. Sections were analyzed at primary magnifications of ×10–100. Apoptosis was evaluated with reference to the two cell types, i.e. neurons (neuronal apoptotic index) and glial cells (glial apoptotic index), and with reference to the sum of the two cell types (cellular apoptotic index). According also to preceding studies on neuronal apoptosis in medullary nuclei (Machaalani & Waters, 2003; Kiryu-Seo et al. 2005), neurons were distinguished from glial cells on the basis of their larger size, clearly defined neuronal cytoplasm, with Nissl substance, and membrane-bound nucleus with a clear nucleolus (Toft et al. 2005). Although it must be considered that the TUNEL method may obliterate some nuclear features which can be used to distinguish these cells, a quite acceptable estimate may be provided by referring to size and cytoplasm characteristics. The optical disector method also allows better evaluation of cytoplasm characteristics, due to the greater thickness of the section and movement of the focal plane. In each section examined by the TUNEL method, all the above-mentioned nuclei were identifiable.
Fig. 1.
(A) Sketch showing varying incidence of neuronal apoptosis in medullary nuclei. (Cu, cuneate nucleus; Ve, medial vestibular nucleus; NTS, nucleus tractus solitarii; DMNV, dorsal motor nucleus of vagus; XII, hypoglossal nucleus; NSTT, nucleus of spinal trigeminal tract; PION, principal inferior olivary nucleus; MION, medial accessory inferior olivary nucleus; DION, dorsal accessory inferior olivary nucleus). (B) Histological section of medulla of an adult case at level of caudal portion of IV ventricle (Klüver-Barrera, 1X). (C) Medullary tegmentum stained by TUNEL method showing higher neuronal apoptosis incidence in Cu, Ve and NSTT, corresponding to lateral area, than in XII, DMNV and NTS. Photomicrographs (10×) mounted together by photo stitch 3.1 (Canon Inc., Lake Success, NY, USA).
A modified version of the optical disector method was used to count cell nuclei (Gundersen et al. 1988; Pakkenberg et al. 1988; West, 1993). The focal plane (or optical section) was moved through the thickness of the section, producing a continuous series of overlapping sections within which counting could be carried out with the following disector counting rules. Counting was performed with high numerical aperture (100×) oil immersion lenses to obtain the smallest possible focal depth. An unbiased counting frame of known area was superimposed on an optical section. The upper right edges of the frame were considered inclusion lines, and the lower left edges were exclusion lines. All cell nuclei seen in focus in the first, most superficial, look-up plane were disregarded. Then all cell nuclei which came into focus through the thickness of the section were counted, including those in the last optical section. The thickness of the upper guard area was 1 µm, so that the disector in which the nuclei were actually counted was 9 µm high. Counting was performed on five sections per case and 20 fields per section for each nucleus. The total numbers of cells corresponding to neuronal ( ), glial (
), glial ( ) and cellular (
) and cellular ( ) nuclei in each field were counted, and the numbers of the corresponding TUNEL-positive cells
) nuclei in each field were counted, and the numbers of the corresponding TUNEL-positive cells  were also recorded.
were also recorded.  and
and  were derived from (
were derived from ( , respectively. The data obtained in each field and section were then summed over the five analyzed sections to provide the total number of cells observed (
, respectively. The data obtained in each field and section were then summed over the five analyzed sections to provide the total number of cells observed ( ) and the number of TUNEL-positive ones (
) and the number of TUNEL-positive ones ( ), respectively.
), respectively.
An estimate of the total numbers of neuronal (NN), glial (NG) and cellular (NC) nuclei and of TUNEL-positive ones (NNA, NGA, NCA) was performed in a two-step process which involved estimating both the numerical densities of the objects, NV, and the volumes of the medullary nuclei, V.
For each medullary nucleus, the densities of the neuronal, glial and cellular nuclei were calculated as follows:
Neuronal density: 
Apoptotic neuronal density: 
Glial density: 
Apoptotic glial density: 
Cellular density: 
Apoptotic glial density: 
Vol(dis) was the volume of each disector, i.e. the product of the area of the counting frame and the height of the disector;  P was the total number of the disectors counted in the specimen.
P was the total number of the disectors counted in the specimen.
For each nucleus, the total numbers of neuronal (NN), glial (NG) and cellular (NC) nuclei were estimated as follows:
Total neuron number: NN = NVN × V
Total apoptotic neuron number: NNA = NVNA × V
Total glial cell number: NG = NVG × V
Total apoptotic glial cell number: NGA = NVGA × V
Total cell number: NC = NVC × V
Total apoptotic cell number: NCA = NVCA × V
V was the volume of each medullary nucleus, estimated using Cavalieri's point-counting method (Gundersen & Jensen, 1987; Gundersen et al. 1999):
where a(p) is the area associated with each sampling point (10 000 or 150 000 µm2, depending on medullary nucleus); T is the mean distance between two consecutive studied sections (0.2 or 0.8 mm, depending on medullary nucleus); n is the number of sections studied for each nucleus; and  is the sum of points hitting a given target.
is the sum of points hitting a given target.
For each nucleus, the apoptotic indices of neurons (INA), glial cells (IGA) and all cells (ICA) were finally estimated as the following ratios:
Neuronal apoptotic index: 
Glial apoptotic index: 
Cellular apoptotic index: 
For each nucleus, mean values (± SD) were calculated for the various sample populations.
Statistics
The Mann–Whitney U-test was performed to verify any differences in apoptotic indices between opiate deaths and controls, SIDS and the other infant cases, and between adults and infants. To reveal differences between the apoptotic indices of the nuclei, statistical analysis was performed in both adult and infant cases using the Kruskal–Wallis test and Dunn's multiple comparison test. In all sample populations, statistical analysis of the linear correlation of apoptotic indices with age and DAI were performed. P < 0.05 was considered to be statistically significant. Statistical calculations were carried out by prism 3.0.3 (GraphPad Software Inc., San Diego, CA, USA).
Results
In all opiate deaths, morphine was detected in blood (range: 0.3–2.7 µg mL−1) and in five cases also in urine (range: 0.2–11.6 µg mL−1). In five cases, alcohol was detected in blood (range: 30–175 mg 100 mL−1) and in four cases also in urine (range: 10–264 mg 100 mL−1). In one case, tetrahydrocannabinol was detected in urine (0.1 µg mL−1).
Apoptotic cells, both neuronal and glial, were found in all medullae examined (Fig. 2). The majority of TUNEL-positive neurons showed morphologic characteristics of apoptosis, such as chromatin condensation and margination and nuclear fragmentation.
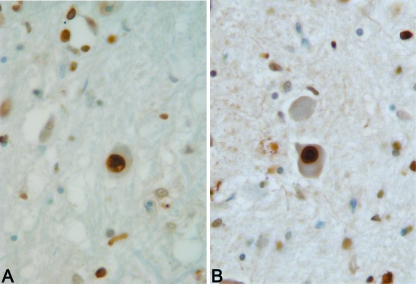
Fig. 2.
TUNEL-positive neurons of inferior olivary complex, showing chromatin condensation (63×).
The Mann–Whitney U-test did not reveal any statistically significant differences in neuronal, glial or cellular apoptotic indices between opiate deaths and controls, or between SIDS and the other infant cases. A statistically significant difference in neuronal apoptotic index was found between adults and infants in the following nuclei: Cu (28.2 ± 16.3% vs. 6.9 ± 8.7%; P < 0.005), Ve (24.7 ± 15.0% vs. 11.3 ± 11.4%; P < 0.05), NTS (11.2 ± 11.2% vs. 2.3 ± 2.4%; P < 0.05), DMNV (6.8 ± 8.5% vs. 0.1 ± 0.2%; P < 0.05) and XII (6.6 ± 5.7% vs. 0.1 ± 0.2%; P < 0.005) (Table 1). Statistically significant differences were not found in cellular or glial apoptotic indices between adults and infants.
Table 1.
Total neuron numbers (NN) and neuronal apoptotic indices (INA) in medullary nuclei of adults and infants (statistically significant differences were not found in glial and cellular apoptotic indices between adults and infants). (Values expressed as mean ± SD)
| Adults | Infants | |||
|---|---|---|---|---|
| NN | INA | NN | INA | |
| XII | 12368 ± 1972 | 6.6 ± 5.7 | 13437 ± 1722(P > 0.05) | 0.1 ± 0.2 (P < 0.005) |
| DMNV | 18051 ± 2623 | 6.8 ± 8.5 | 17063 ± 1391 (P > 0.05) | 0.1 ± 0.2 (P < 0.05) |
| NTS | 111582 ± 16833 | 11.2 ± 11.2 | 119505 ± 16881 (P > 0.05) | 2.3 ± 2.4 (P < 0.05) |
| NSTT | 224416 ± 29686 | 24.4 ± 19.3 | 241139 ± 27242 (P > 0.05) | 20.2 ± 19.0 (P > 0.05) |
| Cu | 22451 ± 2743 | 28.2 ± 16.3 | 20168 ± 2511 (P > 0.05) | 6.9 ± 8.7 (P < 0.005) |
| Ve | 89533 ± 6819 | 24.7 ± 15.0 | 93213 ± 8441 (P > 0.05) | 11.3 ± 11.4 (P < 0.05) |
| PION | 748945 ± 60529 | 12.9 ± 15.0 | 768002 ± 71935 (P > 0.05) | 12.1 ± 21.2 (P > 0.05) |
| MION | 56823 ± 4316 | 10.0 ± 14.4 | 55685 ± 5870 (P > 0.05) | 3.0 ± 6.5 (P > 0.05) |
| DION | 35534 ± 4526 | 13.8 ± 14.3 | 38114 ± 3824 (P > 0.05) | 4.9 ± 7.8 (P > 0.05) |
In both adults and infants, the neuronal apoptotic index of XII was lower than the corresponding glial apoptotic index (P < 0.05). In adults, the neuronal apoptotic indices of NSTT, Cu and Ve were higher than the corresponding glial indices (P < 0.05).
Differences in glial and cellular apoptotic indices between the medullary nuclei were not statistically significant (P > 0.05). Instead, in both adult and infant cases, the Kruskal–Wallis test revealed that the differences in neuronal apoptotic indices between the nuclei reached statistical significance (P < 0.001 and P < 0.005) (Fig. 1C, Fig. 3). In adults, Dunn's multiple comparison test revealed significant differences in comparing the neuronal apoptotic index of Cu with those of PION (P < 0.05), NTS (P < 0.05), MION (P < 0.001), XII (P < 0.001), and DMNV (P < 0.001). The neuronal apoptotic indices of NSTT and Ve were higher than in MION, XII and DMNV (P < 0.05 and P < 0.01, respectively). In infants, the differences between NSTT (20.2 ± 19.0%) and XII (0.1 ± 0.2%) and between NSTT and DMNV (0.1 ± 0.2%) were statistically significant (P < 0.05). Differences in the neuronal apoptotic indices between the other nuclei were not statistically significant (P > 0.05). Table 1 lists the total numbers of neurons of each medullary nucleus.
Fig. 3.
Differences in incidence of neuronal apoptosis in hypoglossal nucleus (A, 20×), dorsal motor nucleus of vagus (B, 20×), cuneate nucleus (C, 20×), medial vestibular nucleus (D, 20×), nucleus of spinal trigeminal tract (E, 20×) and principal inferior olivary nucleus (F, 20×).
No significant statistical correlation of apoptotic indices with age was found in any group. There was no statistically significant difference in the mean DAI between the three groups, and no statistically significant correlation of apoptotic indices with the DAI interval in any group.
Discussion
It has been reported that the TUNEL method can potentially detect random DNA fragmentation due to necrosis (Gold et al. 1994) or post-mortem damage (Scott & Hegyi, 1997). However, in our study, TUNEL-positive cell nuclei did not show any of the characteristics of necrosis, i.e. swelling, dispersion or flocculation of chromosomal material (Clarke, 1990). Moreover, tissue fixation time was quite constant for all specimens, and the apoptotic indices did not correlate with post-mortem delay. Some authors have reported that post-mortem periods of up to 48 h do not influence in situ end-labelling in rat brain (Petito & Roberts, 1995a) and that post-mortem intervals of up to 70 h do not have significant effects on the detection of apoptosis by the TUNEL method in human brain (Adle-Biassette et al. 1995; Gelbard et al. 1995; Petito & Roberts, 1995b; Vincent et al. 1999; Cosenza et al. 2004). However, we must consider that some of the apoptotic phenomena evidenced in our study are to be ascribed to post-mortem changes.
As regards the counting method, we used a modified version of the optical disector. Large objects have a greater probability of being hit by a single section than small ones, so that an object seen in a single section is a biased, non-representative sample of all objects (Gundersen et al. 1988; Pakkenberg et al. 1988). In particular, this is true for nuclei which may change in size, like apoptotic nuclei. Instead, the probability of an object being hit by a section but not by a parallel section is the same for both large and small objects (Pakkenberg et al. 1988). The physical and optical disector methods are based on these unbiased principles. In the present work, we used an optical disector, due to its simpler and less time-consuming procedure, moving the focal plane through the thickness of the section and counting the nuclei which came into view or disappeared. The optical disector method also allows better evaluation of cell characteristics, as the focal plane moves in a thicker section, and facilitates distinctions between neuronal and glial cells. Nevertheless, it must be noted that in our study the sections were not thick enough to eliminate the above bias completely.
In the present study, differences in apoptotic indices between opiate deaths and controls were analyzed, as opiates have been shown to induce apoptosis of neuronal cells in experimental studies in rats (Atici et al. 2004), chick embryo cell cultures (Goswami et al. 1998), and murine (Goswami et al. 1998) and human (Yin et al. 1997; Hu et al. 2002) neuroblastoma cell lines. It has also been suggested that long-term neurobehavioural damage (Steingart et al. 2000) and developmental abnormalities (Basheer et al. 1992; Seatriz & Hammer, 1993; Harlan & Song, 1994; Malanga & Kosofsky, 1999) in the brains of neonatal animals born to pregnant animals exposed to opiates may be ascribed to morphine-induced apoptosis of neurons (Hu et al. 2002). In our study, comparison between apoptotic indices of opiate deaths and controls did not show statistically significant differences. Thus, the in vivo apoptotic effect of morphine was not evidenced in our study. This finding may be ascribed to post-mortem changes or to a common effect due to terminal hypoxic-ischaemic injury. Opiate deaths and controls shared systemic hypoxic-ischaemic injury as the terminal cause of death, and the literature has clarified that this kind of injury may produce neuronal apoptosis (Snider et al. 1999).
As regards the infant cases, a higher incidence of neuronal apoptosis within the brainstem of SIDS victims has been described, with particular reference to the gracile nucleus, Cu, NSTT, NTS, lateral reticular formation and lateral cuneate nucleus, and ascribed to hypoxia (Waters et al. 1999). In our study, however, we did not find higher apoptotic indices in SIDS cases with respect to other infant cases, although this may be due to the small number of cases.
A comparison was also made between adults and infants, due to the absence of significant internal differences in the two populations. Glial and cellular apoptosis indices did not show statistically significant differences between adults and infants. Instead, the neuronal apoptotic indices of infants were lower than those of adults, with statistical significance for XII, DMNV, Cu, Ve and NTS. These findings indicate that neuronal populations and not glial ones show different characteristics of survival between adults and infants. The neurons of these infant nuclei may be more resistant to hypoxic-ischaemic injury or post-mortem changes. Instead, no statistically significant correlation between age and apoptosis was found in the adult series.
Heterogeneity was found among the neuronal apoptotic indices of the various nuclei of the medulla oblongata, whereas the glial and cellular apoptotic indices did not show statistically significant differences. The topographical pattern of apoptosis in the medulla oblongata of adults did not differ significantly from that of infants. These findings may be explained by reference to differing hypotheses. It may be that neurons of different nuclei simply take different times to become apoptotic, as a result of post-mortem changes. Otherwise, these findings may indicate the different vulnerability of the medullary nuclei in response to hypoxic-ischaemic injury. The fact that apoptosis of glial cells, which have higher resistance than neurons to hypoxic-ischaemic injury, did not vary between the medullary nuclei supports the hypothesis of the different vulnerability of neurons to in vivo injury. Further studies on experimental animals will be necessary to verify this hypothesis.
Three areas of vascularization have been described in the medulla: median, fed by the paramedian arteries; middle, fed by the short circumferential arteries; and lateral, fed by the posterior inferior cerebellar artery (PICA) (Foix & Hillemand, 1925; Lazorthes, 1961). Although at the moment we cannot evaluate the apoptotic component due to post-mortem changes, it is intriguing that the nuclei with higher neuronal apoptotic indices and with neuronal apoptosis indices higher than glial ones, i.e. Cu, Ve and NSTT, are all located in the lateral area of the medullary tegmentum and share the same vascular supply from the PICA, thus suggesting that local vascularization plays a role in determining the spatial distribution of apoptosis.
Acknowledgments
Part of this study was presented at the Summer Meeting of the Anatomical Society of Great Britain and Ireland, hosted at the Department of Anatomy, University of Cardiff, July 5–7, 2005.
References
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, et al. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Hirsch EC, Agid Y. Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol. 1997;12:603–610. [PubMed] [Google Scholar]
- Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, et al. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004;114:1001–1011. doi: 10.1080/00207450490461314. [DOI] [PubMed] [Google Scholar]
- Basheer R, Yang J, Tempel A. Chronic prenatal morphine treatment decreases G alpha s mRNA levels in neonatal frontal cortex. Brain Res Dev Brain Res. 1992;70:145–148. doi: 10.1016/0165-3806(92)90113-b. [DOI] [PubMed] [Google Scholar]
- Chan WY, Yew DT. Apoptosis and bcl-2 oncoprotein expression in the human fetal central nervous system. Anat Rec. 1998;252:165–175. doi: 10.1002/(SICI)1097-0185(199810)252:2<165::AID-AR2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Lee SC. HIV-1 expression protects macrophages and microglia from apoptotic death. Neuropath Appl Neurobiol. 2004;30:478–490. doi: 10.1111/j.1365-2990.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- Dambska M, Dydyk L, Szretter T, Wozniewicz J, Myers RE. Topography of lesions in newborn and infant brains following cardiac arrest and resuscitation: damage to brainstem and hemispheres. Biol Neonate. 1976;29:194–206. doi: 10.1159/000240864. [DOI] [PubMed] [Google Scholar]
- De Caro R, Parenti A, Montisci M, Guidolin D, Macchi V. Solitary tract nuclei in acute heart failure. Stroke. 2000;31:1187–1193. doi: 10.1161/01.str.31.5.1187. [DOI] [PubMed] [Google Scholar]
- De Caro R, Parenti A, Montisci M, Guidolin D, Macchi V. Symmetrical selective neuronal necrosis in solitary tract nuclei. Int J Legal Med. 2003;117:253–254. doi: 10.1007/s00414-003-0389-0. [DOI] [PubMed] [Google Scholar]
- Foix C, Hillemand P. Les artères de l’axe encéphalique jusqu’au diencéphale inclusivement. Rev Neurol. 1925;11:705–739. [Google Scholar]
- Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, et al. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Gilles FH. Hypotensive brain stem necrosis: selective symmetrical necrosis of tegmental neuronal aggregates following cardiac arrest. Arch Pathol. 1969;88:32–41. [PubMed] [Google Scholar]
- Gold R, Schimied M, Giegerich G, Breitschopf H, Hartung HP, Tovka KV, et al. Differentation between cellular apoptosis and necrosis by combined use of in situ tailing and nick translation techniques. Lab Invest. 1994;71:219–225. [PubMed] [Google Scholar]
- Goswami R, Dawson SA, Dawson G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evan SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EBV, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology-reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Song DD. Prenatal morphine treatment and the development of the striatum. Regul Pept. 1994;54:117–118. [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Friede RL. Hypotensive brain stem necrosis or cardiac arrest encephalopathy? Acta Neuropathol. 1980;50:53–56. doi: 10.1007/BF00688534. [DOI] [PubMed] [Google Scholar]
- Kerr JFT, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryu-Seo S, Hirayama T, Kato R, Kiyama H. Noxa is a critical mediator of p53-dependent motor neuron death after nerve injury in adult mouse. J Neurosci. 2005;25:1442–1447. doi: 10.1523/JNEUROSCI.4041-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazorthes G. Vascularisation et Circulation Cérébrales. Paris: Masson & Cie Editeurs; 1961. [Google Scholar]
- Leech RW, Alvord EC., Jr Anoxic-ischaemic encephalopathy in the human neonatal period: the significance of brain stem involvement. Arch Neurol. 1977;34:109–113. doi: 10.1001/archneur.1977.00500140063013. [DOI] [PubMed] [Google Scholar]
- Machaalani R, Waters KA. Increased neuronal cell death after intermittent hypercapnic hypoxia in the developing piglet brainstem. Brain Res. 2003;985:127–134. doi: 10.1016/s0006-8993(03)03003-8. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Mechanisms of action of drugs of abuse on the developing fetal brain. Clin Perinatol. 1999;26:17–37. [PubMed] [Google Scholar]
- McRitchie DA, Tork I. The internal organization of the human solitary nucleus. Brain Res Bull. 1993;31:171–193. doi: 10.1016/0361-9230(93)90024-6. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Parenti A, Macchi V, Snenghi R, Porzionato A, Scaravilli T, Ferrara SD, et al. Selective stroke of the solitary tract nuclei in two cases of central sleep apnoea. Clin Neuropathol. 2005;24:239–246. [PubMed] [Google Scholar]
- Paxinos G, Huang XF. Atlas of the Human Brainstem. San Diego: Academic Press; 1995. [Google Scholar]
- Petito CK, Roberts B. Effect of post-mortem interval on in situ end-labeling of DNA oligonucleosomes. J Neuropathol Exp Neurol. 1995a;54:761–765. doi: 10.1097/00005072-199511000-00002. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995b;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Pindur J, Capin DM, Johnson MI, Rance NE. Cystic brainstem necrosis in a premature infant after prolonged bradycardia. Acta Neuropathol. 1992;83:667–669. doi: 10.1007/BF00299419. [DOI] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Ferrara SD, Parenti A, De Caro R. C-FOS expression in the subnucleus gelatinosus of the human nucleus tractus solitarii. Ital J Anat Embryol. 2004a;109:125–134. [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Parenti A, De Caro R. The distribution of mast cells in the human area postrema. J Anat. 2004b;204:141–147. doi: 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Morsut L, Parenti A, De Caro R. Microvascular patterns in human medullary tegmentum at the level of the area postrema. J Anat. 2005;206:405–410. doi: 10.1111/j.1469-7580.2005.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz T, Geddes JF. Symmetrical columnar necrosis of the basal ganglia and brain stem in an adult following cardiac arrest. Clin Neuropathol. 1988;6:294–298. [PubMed] [Google Scholar]
- Scott RJ, Hegyi L. Cell death in perinatal hypoxic-ischaemic brain injury. Neuropathol App Neurobiol. 1997;23:307–314. [PubMed] [Google Scholar]
- Seatriz JV, Hammer RP., Jr Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30:523–527. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci. 1999;893:243–253. doi: 10.1111/j.1749-6632.1999.tb07829.x. [DOI] [PubMed] [Google Scholar]
- Steingart RA, Abu-Roumi M, Newman ME, Silverman WF, Slotkin TA, Yanai J. Neurobehavioral damage to cholinergic systems caused by prenatal exposure to heroin or phenobarbital: cellular mechanisms and the reversal of deficits by neural grafts. Brain Res Dev Brain Res. 2000;122:125–133. doi: 10.1016/s0165-3806(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Roessman U. Hypotensive brainstem necrosis in a stillborn. Acta Neuropathol. 1984;65:166–167. doi: 10.1007/BF00690472. [DOI] [PubMed] [Google Scholar]
- Toft MH, Gredal O, Pakkenberg B. The size distribution of neurons in the motor cortex in amyotrophic lateral sclerosis. J Anat. 2005;207:399–407. doi: 10.1111/j.1469-7580.2005.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent VAM, De Groot CJ, Lucassen PJ, Potegies P, Troost D, Tilders FJ, et al. Nitric oxide synthase expression and apoptotic cell death in brains of AIDS and AIDS dementia patients. AIDS. 1999;13:317–326. doi: 10.1097/00002030-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Waters KA, Meehan B, Huang JQ, Gravel RA, Michaud J, Cote A. Neuronal apoptosis in sudden infant death syndrome. Pediatr Res. 1999;45:166–172. doi: 10.1203/00006450-199902000-00002. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Wyllie A. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yin DL, Ren XH, Zheng ZL, Pu L, Jiang LZ, Ma L, et al. Etorphine inhibits cell growth and induces apoptosis in SK-N-SH cells: involvement of pertussis toxin-sensitive G proteins. Neurosci Res. 1997;29:121–127. doi: 10.1016/s0168-0102(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Zhang F, Yin W, Chen J. Apoptosis in cerebral ischemia: executional and regulatory signalling mechanisms. Neurol Res. 2004;26:835–845. doi: 10.1179/016164104X3824. [DOI] [PubMed] [Google Scholar]