Figure 1.
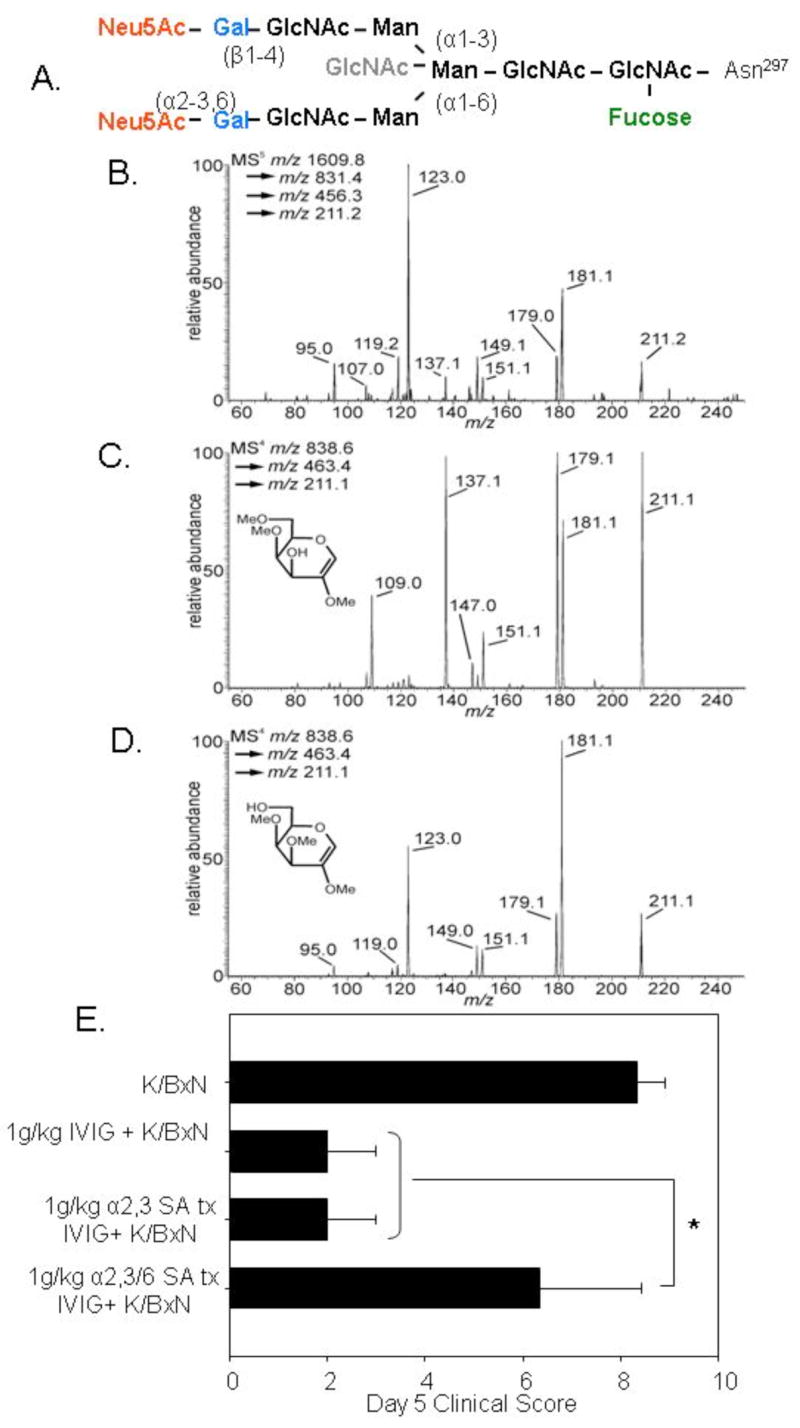
α2,6 linkages are the predominant sialic acid linkage on IVIG Fc glycans. A. The IgG Fc glycan is a bisecting core of seven sugars (black), and can vary at a number of positions by the addition of fucose (green) to the core, a bisecting GlcNAc (gray), or by addition of galactose (blue) and sometimes sialic acid (red) to the arms. B. Sequential mass spectrometry analysis of SNA-enriched IVIG Fc glycans was performed to determine the sialic acid linkage type and relative proportion of the linkages in the active component of IVIG. The resulting footprint of the B/Y galactosyl fragment monomer derived from the SNA-enriched Fc glycan was compared to the analogous B/Y fragments from (C) 2,3 sialyllactose and (D) 2,6 sialyllactose standards. The spectrum generated from the SNA+ IVIG Fc glycan (B) most closely matches that of 2,6 sialyllactose (D), particularly with respect to the m/z 123 and 95 fragments, and the much smaller abundances of m/z 137 and 109 ions. Next, IVIG was treated with linkage-specific sialidases to remove only 2,3 (α2,3 SA) or both 2,3 and 2,6 (α2,3/6 SA) sialic acids. E. The sialidase-treated IVIG preparations were administered to mice prior to K/BxN sera, and footpad swelling was monitored over the next seven days and recorded as clinical scores. Mean and standard deviation of 5 mice per group 5 days post treatment are plotted; *denotes p<0.05 as determined by an Anova test followed by Tukey post hoc test.
