Figure 4.
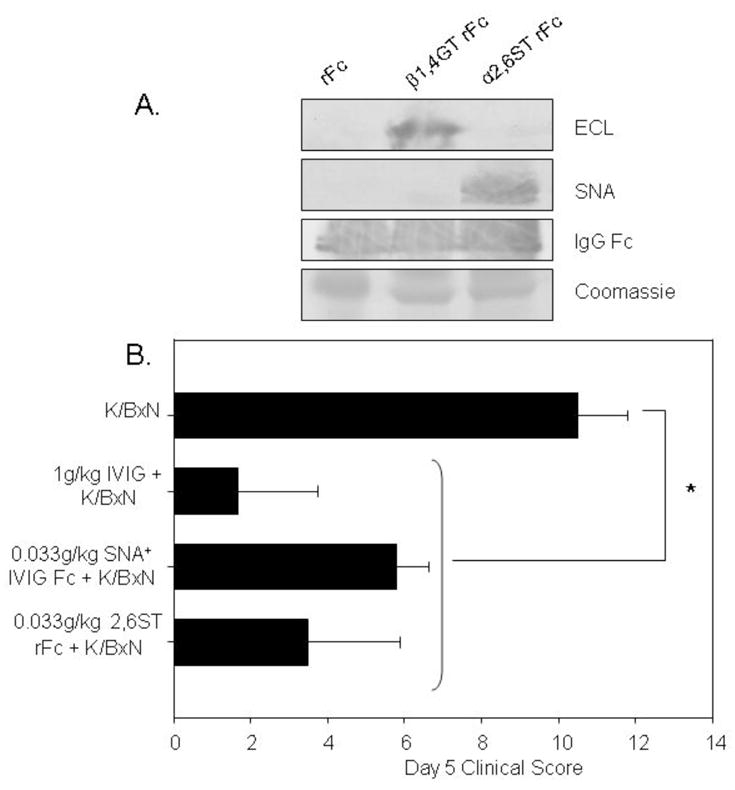
Recombinant, sialylated IgG Fc fragments are anti-inflammatory. Recombinant human IgG1 was digested with papain and Fcs were purified by HPLC followed by protein G purification. The recombinant Fcs (rFc) were galactosylated and sialylated in vitro with α2,6 sialyltransferase. A. Glycosylation was confirmed by lectin blotting for terminal galactose with ECL (top panel), α2,6 sialic acid with SNA (middle panel), and coomassie loading controls are shown in the bottom panel. B. Mice were administered 1g/kg IVIG, 0.033g/kg SNA+ IVIG Fcs, or 0.33g/kg sialylated rFc (2,6ST rFc) 1 hour prior to K/BxN sera, and footpad swelling was monitored over the next several days. Mean and standard deviation of clinical scores of 4–5 mice per group are plotted; *denotes p<0.05 as determined by Kruskal-Wallis Anova followed by Dunn’s post hoc.
