FIG. 1.
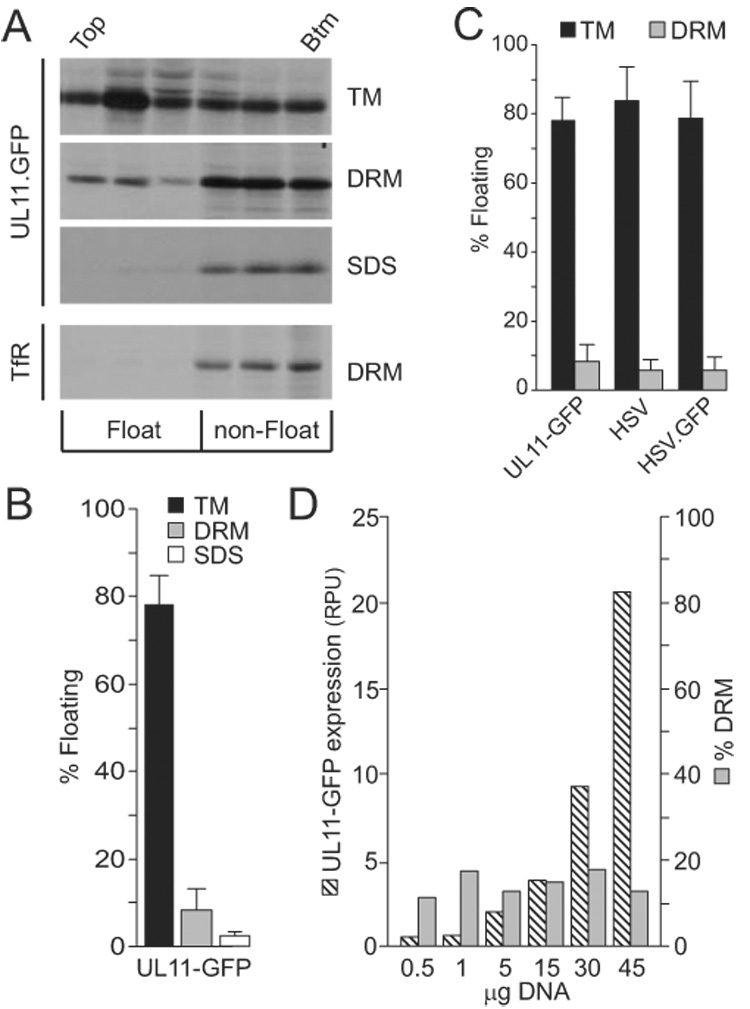
Flotation analysis of UL11. (A) A7 cells transfected with a UL11-GFP expression vector were metabolically labeled for 2.5 h and osmotically disrupted, as described in the text. Cytoplasmic membranes were treated with nothing (TM, total membranes), 0.5% TX-100 (DRM, detergent-resistant membranes), or 0.5% SDS (negative control). The ability of UL11-GFP to float to the upper fractions of sucrose step gradients during centrifugation was examined, and representative autoradiograms, obtained following immunoprecipitation and SDS-PAGE analysis, are shown. As a control for DRM disruption, endogenous transferrin receptor (TfR) was monitored in one experiment following radiolabeling, TX-100 treatment, and flotation. The tops and bottoms of the gradients are indicated. (B) Phosphorimager analysis was used to quantitate the flotation results, which are shown as the percentage of floating protein (top three fractions) relative to the total protein (all fractions). The averages of four experiments are shown, along with the standard deviations. (C) Flotation assays were used to compare the membrane-binding properties of radiolabeled UL11-GFP produced in transfected cells with untagged UL11 and UL11-GFP produced by wild-type and recombinant viruses (HSV and HSV.GFP, respectively). Cells were labeled for the final 2.5 h of infection, harvested, and floated as described in the text. The averages from at least four experiments are shown, along with the standard deviations. (D) To examine the saturability of DRMs, cells were transfected with increasing amounts of plasmid DNA, metabolically labeled, and subsequently analyzed for UL11-GFP expression levels (hatched bars; RPU = relative phosphorimager units) and DRM localization (grey bars). A repeat of this experiment gave comparable results.
