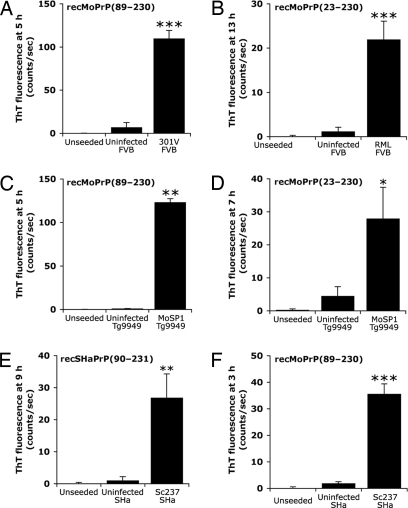
Fig. 2.
The amyloid seeding effect occurs with several different prion strains isolated from wild-type mice, Tg mice, and Syrian hamsters, and with several different recombinant PrP substrates. Each strain was purified by PTA precipitation of brain homogenates. The following prion strains successfully seeded amyloid formation from the following substrates: 301V for recMoPrP(89–230) (A); RML for full-length recMoPrP(23–230) (B); MoSP1 for both recMoPrP(89–230) (C) and full-length recMoPrP(23–230) (D); and Sc237 for both recSHaPrP(90–231) (E) and recMoPrP(89–230) (F). As controls, no seeds and uninfected, PTA-precipitated brain homogenates of the respective animals were added. For all experiments, seeding significantly reduced the lag phase compared with controls (n = 6; *, P < 0.01; **, P < 0.05; ***, P < 0.001). Bars denote standard error.