Fig. 2.
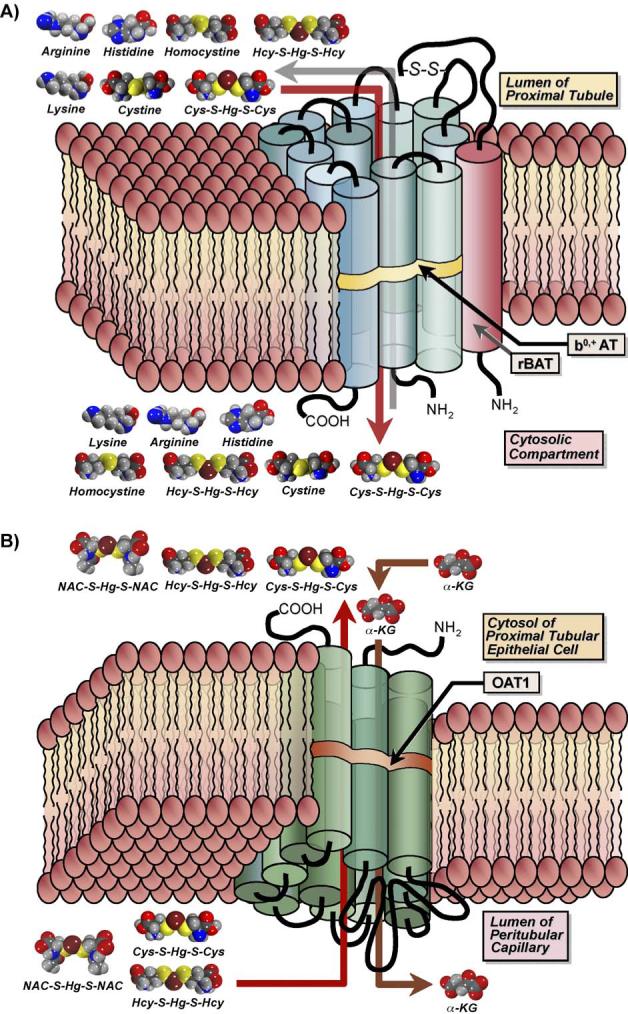
Diagrammatic representation of the transport of amino acids and mercuric conjugates of amino acids by the amino acid transporter, system b0,+ (A) and the organic anion transporter 1 (OAT1; B). (A) System b0,+ is a Na+-independent transporter comprised of a heavy chain and a light chain, which are linked together by a disulfide bond (S–S). The light chain, b0,+ AT (blue cylinders), possesses 12 transmembrane domains, while the heavy chain, rBAT (red cylinder), traverses the plasma membrane only once. This carrier is localized in the luminal plasma membrane of transporting epithelia (such as renal proximal tubular epithelial cells) and functions as an amino acid exchanger that mediates the transport of cystine as well as a variety of neutral and cationic amino acids. Recent studies have identified additional substrates for this carrier, including mercuric conjugates of cysteine (Cys; Cys-S-Hg-S-Cys) and homocysteine (Hcy; Hcy-S-Hg-S-Hcy), which are similar structurally to the amino acids cystine and homocystine, respectively. Experiments carried out in Madin-Darby canine kidney (MDCK) cells transfected stably with both subunits of system b0,+ showed that Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy mimic cystine and homocystine, respectively, at the site of this transporter. (B) The organic anion transporter 1 is a multi-specific carrier that is localized in the basolateral plasma membrane of many types of epithelial cells. Its expression is especially pronounced in renal proximal tubular epithelial cells. This transporter spans the plasma membrane 12 times and has two large intracellular loops, with the first between the first and second transmembrane domains and the second joining the sixth and seventh domains. The inward transport of organic anions is driven by the outward flux of α-ketoglutarate (α-KG). Data from recent studies in which MDCK cells were transfected stably with OAT1 demonstrate that mercuric conjugates of N-acetylcysteine (NAC-S-Hg-S-NAC), Cys-S-Hg-S-Cys, and Hcy-S-Hg-S-Hcy are transportable substrates of this carrier. As hypothesized for system b0,+, these mercuric species likely act as molecular mimics of endogenous substrates of OAT1.
