Figure 7. Histidine and tryptophan starvation induces mRNA cleavage and tmRNA-mediated peptide tagging activities.
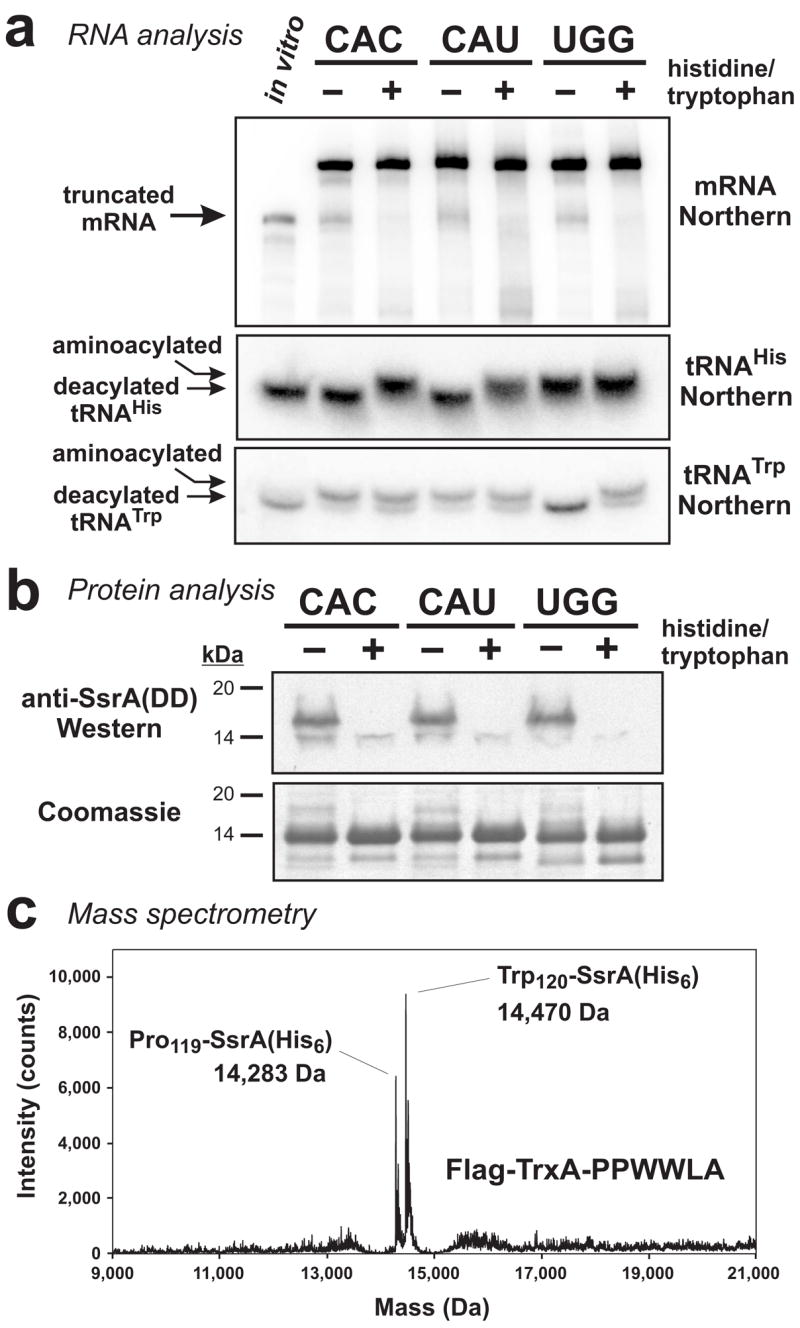
(a) Northern blot analyses of RNA isolated from ΔtmRNA cells that were fed (+) or starved (−) for histidine or tryptophan. Truncated mRNA from the CAC and CAU containing constructs was only produced during histidine starvation, and tryptophan starvation induced cleavage of the UGG containing mRNA. The position of truncated mRNA indicated by the arrow corresponds to a control in vitro transcript of flag-trxA(PPHHLA)CAU that was truncated after the first CAU codon. RNA was resolved at low pH on acid-urea gels for Northern analysis of tRNAHis and tRNATrp. Under these electrophoresis conditions, aminoacylated tRNAs were resolved from deacylated tRNAs. The sample loaded in the in vitro lane contained 0.2 pmole of truncated in vitro transcript mixed with 10 μg of total RNA that had been alkali-treated to deacylate tRNA. (b) Flag-TrxA-PPHHLA and Flag-TrxA-PPWWLA proteins were produced in tmRNA(DD) cells under amino acid-fed (+) or amino acid-starved (−) conditions. Total protein was analyzed by SDS-PAGE followed by Coomassie blue staining, and by Western blot analysis using antibodies specific for the SsrA(DD) peptide tag. SsrA(DD) peptide tag addition was only observed during amino acid starvation. (c) Flag-TrxA-PPWWLA was expressed in tmRNA(His6) cells under tryptophan starvation conditions and purified by Ni2+ affinity chromatography. Mass spectrometry detected two species corresponding to SsrA(His6) peptide tag addition after Pro-119 (calculated mass, 14,283.1 Da) and Trp-120 (calculated mass, 14,469.3 Da).
