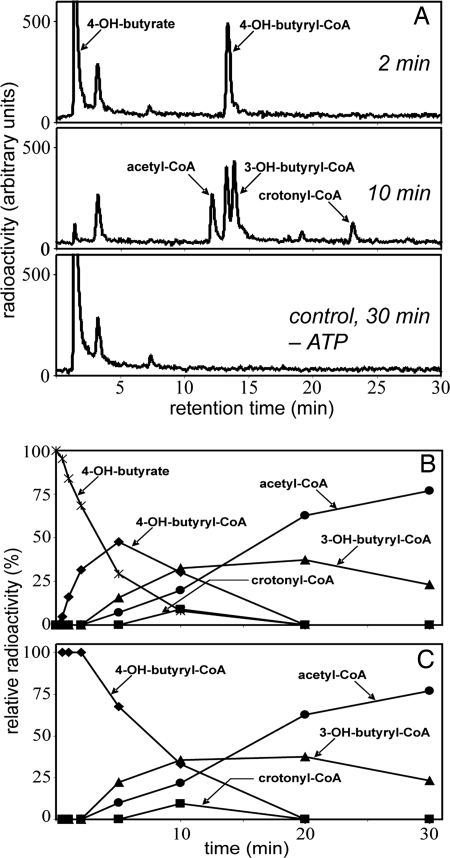
Fig. 1.
Conversion of [1-14C]4-hydroxybutyrate to [14C]acetyl-CoA at 60°C by cell extracts of I. hospitalis. (A) HPLC separation of labeled substrate and products and 14C detection by flow-through scintillation counting. The figure shows samples taken after 2- and 10-min incubation, as well as a control experiment lacking ATP after 30-min incubation. The radioactive peak at 3.5 min most likely represents γ-butyrolactone, which forms spontaneously from 4-hydroxybutyrate at acidic pH or from 4-hydroxybutyryl-CoA at neutral pH. (B) Time course of substrate consumption and product formation. 100% corresponds to the total radioactivity added at the beginning. (C) Percentage of radioactivity present in the individual products, compared with the total radioactivity in all labeled products, versus time. 100% corresponds to the total radioactivity contained in all products at a given time. The strong negative slope for 4-hydroxybutyryl-CoA indicates that it is the first intermediate. The strong positive slope for acetyl-CoA indicates that it is the end product. Crotonyl-CoA and 3-hydroxybutyryl-CoA behave like intermediates between 4-hydroxybutyryl-CoA and acetyl-CoA.