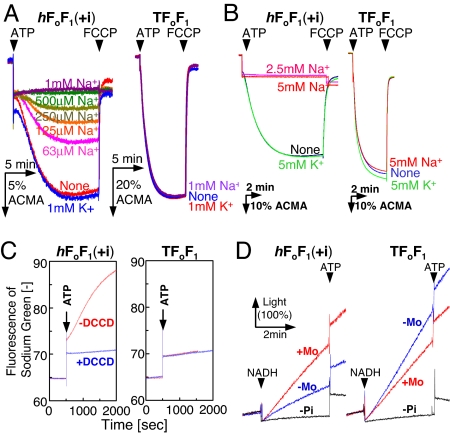
Fig. 2.
Activities of the hybrid F1Fo (Left) compared with TF1Fo (Right). (A) H+-pumping activity of inverted E. coli membrane vesicles containing the hybrid F1Fo [hF1Fo(+i)] and TF1Fo. NaCl or KCl was added into the assay mixture at the concentrations indicated. The reaction was initiated by adding 1 mM K+-ATP and terminated with FCCP. %ACMA shows the fluorescent intensity of ACMA, in which the initial intensity before the addition of ATP is calibrated as 100%. (B) H+-pumping activity of PLs containing hF1Fo(+i) and TF1Fo. The assay conditions were the same as A. (C) Na+-pumping activity of PLs containing hF1Fo(+i) and TF1Fo. As indicated, PLs were reacted with 50 μM DCCD for 1 h and used for the measurement. The reaction was initiated by the addition of 1.3 mM Na+2-ATP, and the increase in Na+ concentration inside the PLs was monitored with an increase in sodium green fluorescence at 540 nm. (D) ATP synthesis activity of membrane vesicles containing hF1Fo(+i) and TF1Fo. Electrochemical potential of H+ was generated by respiratory H+ pumps in the membrane vesicles driven by NADH oxidation. ATP synthesis was monitored in real time at 35°C by luciferase reaction at 560 nm. As indicated, a Na+/H+ antiporter, monensin (5 μM) was added to convert some amount of H+ gradient into Na+ gradient. Traces indicated by “-Pi” represent negative controls without addition of Pi. Light (100%) in D corresponds to the initial luminescence intensity in the cuvette before the addition of NADH.