Introduction
Recently, cholesterol-independent effects of HMG-CoA reductase inhibitors (statins) have been clarified, such as modulation of cell morphology and/or cell-substrate attachment [1]. Some of these effects are suggested to be mediated by intermediate compounds of the cholesterol synthesis, i.e., the isoprenoids [2].
Here, we investigated the effect of HMG-CoA reductase inhibitor and/or the related compounds of the cholesterol synthesis pathway on collagen gel-contractility, cell morphology, and/or cell-substrate attachment of hepatic stellate cells (HSCs), using myofibroblast-like stellate cell line derived from a CCl4-induced cirrhotic rat liver.
Methods
Materials
Simvastatin [3] was kindly provided by Merck & Co., Inc. (Rahway, NJ) and was converted to open acid form before use. Farnesylpyrophosphate (FPP) and geranylgeranyl-pyrophosphate (GGPP) were obtained from Sigma Chemical Co. (St. Louis, MO). Prenyltransferase inhibitors; FTI-277, and GGTI-286, were from Calbiochem (La Jolla, CA). A monoclonal antibody against phosphorylated serine 19 of myosin regulatory light chain (anti-MLC-pS19) was previously described [4]. A monoclonal antibody against RhoA was from Santa Cruz Biotechnology (Santa Cruz, CA). Other materials were purchased commercially.
Cell culture
The hepatic myofibroblast-like stellate cell line, CFSC-8B (CFSC), derived from a CCl4-induced cirrhotic rat liver [5], was maintained in DMEM and was supplemented with 10% fatal calf serum (FCS).
Collagen gel-contraction assay
Contractility of CFSCs was evaluated using collagen gel lattices on 24-well culture plates as described previously [4].
F-actin staining
For visualization of F-actin, the cells were stained overnight with TRITC-labeled phalloidin (Sigma), and then they were observed by fluorescence microscopy.
Cell adhesion assays
Cell adhesion was measured using the electric cell-substrate impedance sensor system (ECIS; Applied BioPhysics, Inc., Troy, NY) as described previously [4].
Immunoblotting
Cellular proteins were separated by SDS-PAGE, and immunoblotted using anti-MLC-pS19 or anti-RhoA. Immunoreactive proteins were visualized using a chemiluminescence kit (Amersham).
Statistical analysis
Data were given as the mean value with the standard error of it, and were analyzed by the paired Student's t test.
Results and Discussion
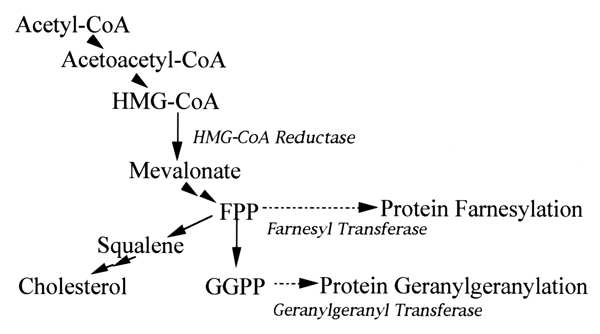
HMG-CoA reductase inhibitors are widely used in patients with liver disease, such as fatty liver and/or primary liver cirrhosis, however, the effects and the mechanisms of these inhibitors on the liver remain uncertain as well in HSCs [6,7]. They block the conversion of HMG-CoA to mevalonate, the rate-limiting step in the synthesis of cholesterol, moreover, several recent studies have been focused on their cholesterol-independent effects. By modulating the initial part of the cholesterol synthesis pathway, they decrease the level of numerous important intermediate compounds including isoprenoids including FPP and GGPP (Figure 1). Isoprenoids are lipid attachments involved in post-translational modification of some proteins such as gamma-subunit of the heterotrimeric G proteins, the small G proteins as Ras, Rho, Rap, Rab, or Ral [2]. Thus, they can modulate various biological or physiological mechanisms.
Figure 1.
The cholesterol synthetic pathway.
In the present study we found that the addition of 10-5M of simvastatin attenuated the contractile activity of collagen-gel by CFSCs, which was recovered by co-addition of 10-3M of mevalonate, the direct metabolite of HMG-CoA. The inhibitory effect of simvastatin was also cancelled by co-addition of 10-5M of GGPP, but not by 10-5M of FPP or squalene, the late step product of the cholesterol synthesis. Moreover, the inhibitory effect was partially reproduced by addition of 10-5M of GGTI, not by 10-5M of FTI. Next, we found that cell morphology and/or the formation of stress fibers of CFSCs by F-actin staining were abrogated by simvastatin, which were maintained in the presence of mevalonate, and GGPP, but not of FPP. They were also attenuated in the presence of GGTI. We revealed further that the adhesive area of CFSCs to extracellular substrate by ECIS were reduced by simvastatin and GGTI, which were maintained in the presence of mevalonate, and GGPP. The above observations may suggest that HMG-CoA inhibitor modulates the morphological and cytoskeletal changes through the dynamic reorganization of actin filaments, and the cell-extracellular matrix interaction, resulting in the attenuation of the contraction of collagen gel lattices, and that protein geranylgeranylation is involved in this mechanism. Protein prenylation of RhoA is required to functional activities of RhoA [2]. Western blotting analyses showed that phosphorylated myosin regulatory light chain and prenylated RhoA were maintained in the presence of mevalonate, and GGPP, which were attenuated in the presence of simvastatin and/or GGTI. It may be suggested that prenylated RhoA might be associated with collagen-gel contractility, cell morphology, and/or cell-substrate attachment of CFSCs exerted by simvastatin and/or the isoprenoids.
In conclusion, HMG-CoA reductase inhibitor may modulate CFSC morphology, its attachment to surrounding extracellular matrix and its contraction by a mechanism involving protein geranylgeranylation.
Acknowledgments
Acknowledgements
This work was supported in part by Grant RO1AA10541 from the National Institute of Alcohol Abuse and Alcoholism (MR). The opinions or assertions contained herein are the private views of the Authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Contributor Information
Mikio Yanase, Email: yanase-1IM@h.u-tokyo.ac.jp.
Hitoshi Ikeda, Email: ikeda-1IM@h.u-tokyo.ac.jp.
Atsushi Matsui, Email: atsushim@saitama-med.ac.jp.
Eisei Noiri, Email: noiri-1IM@h.u-tokyo.ac.jp.
Tomoaki Tomiya, Email: tomiya-1IM@h.u-tokyo.ac.jp.
Masahiro Arai, Email: arai-1IM@h.u-tokyo.ac.jp.
Yukiko Inoue, Email: yanase-1IM@h.u-tokyo.ac.jp.
Kazuaki Tejima, Email: yanase-1IM@h.u-tokyo.ac.jp.
Kayo Nagashima, Email: yanase-1IM@h.u-tokyo.ac.jp.
Takako Nishikawa, Email: yanase-1IM@h.u-tokyo.ac.jp.
Satoshi Kimura, Email: yanase-1IM@h.u-tokyo.ac.jp.
Kenji Fujiwara, Email: yanase-1IM@h.u-tokyo.ac.jp.
Marcos Rojkind, Email: marcos.rojkind@na.amedd.army.mil.
Itsuro Ogata, Email: itsuro@viola.ocn.ne.jp.
References
- Liu L, Moesner P, Kovach NL, Bailey R, Hamilton AD, Sebti SM, Harlan JM. Integrin-dependent leukocyte adhesion involves geranylgeranylated protein(s) J Biol Chem. 1999;274:33334–33340. doi: 10.1074/jbc.274.47.33334. [DOI] [PubMed] [Google Scholar]
- Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- Parker RA, Clark RW, Sit SY, Lainer TL, Grosso RA, Wright JJK. Selective inhibition of cholesterol synthesis in liver versus extrahepatic tissues by HMG-CoA reductase inhibitors. J Lipid Res. 1990;31:1271–1282. [PubMed] [Google Scholar]
- Yanase M, Ikeda H, Matsui A, Maekawa H, Noiri E, Tomiya T, Arai M, Yano T, Shibata M, Ikebe M, Fujiwara K, Rojkind M, Ogata I. Lysophosphatidic acid enhances collagen-gel contraction by hepatic stellate cells: Association with Rho-kinase. Biochem Biophys Res Commun. 2000;277:72–78. doi: 10.1006/bbrc.2000.3634. [DOI] [PubMed] [Google Scholar]
- Greenwel P, Rubin J, Schwarz M, Hertzberg EL, Rojkind M. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterologous with regard to proliferation, expression of extracellular matrix components, interleukin-6 and connexin-43. Lab Invest. 1993;69:210–216. [PubMed] [Google Scholar]
- Mallat A, Pr–aux AM, Blazejewski S, Dhumeaux D, Rosenbaum J, Mavier P. Effect of simvastatin, an inhibitor of hydroxy-methylglutaryl coenzyme A reductase, on the growth of human Ito cells. Hepatology. 1994;19:1095–1099. doi: 10.1016/0270-9139(94)90855-9. [DOI] [PubMed] [Google Scholar]
- Carloni V, Pinzani M, Giusti S, Romanelli RG, Parola M, Bellomo G, Failli P, Hamilton AD, Sebti SM, Laffi G, Gentilini P. Tyrosine phosphorylation of focal adhesion kinase by PDGF is dependent on Ras in human hepatic stellate cells. Hepatology. 2000;31:131–140. doi: 10.1002/hep.510310121. [DOI] [PubMed] [Google Scholar]