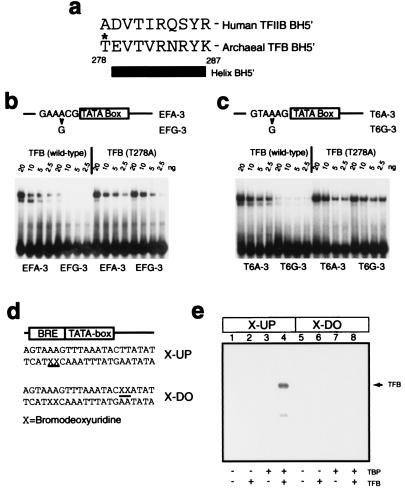
Figure 5.
The orientation of the archaeal TBP/TFB/DNA complex is the same as that of the eukaryal ternary complex. (a) Amino acid sequence of helix BH5′ and preceding residue in human TFIIB and archaeal TFB. The T278 residue is indicated with an asterisk. (b) EMSAs employing double-stranded oligonucleotides corresponding to the wild-type ef1α promoter (EFA-3) or ef1α promoter with an A⋅T to G⋅C substitution 3 base pairs upstream from the TATA box (EFG-3). Reaction mixtures were incubated with 20 ng of TBP and the indicated amount of TFB or TFB (T278A). (c) EMSAs employing double-stranded oligonucleotides corresponding to the wild-type T6 promoter (T6A-3) or T6 promoter with an A⋅T to G⋅C substitution 3 base pairs upstream from the TATA box (T6G-3). Reaction mixtures were incubated with 20 ng of TBP and the indicated amount of TFB or TFB (T278A). (d) Partial sequence of probes used in UV crosslinking experiments. The positions of 5-bromodeoxyuridine (BrdUrd) substitutions for thymidine are indicated by X and underlined. (e) The presence of BrdUrd substitutions upstream of the TATA box allows UV-mediated crosslinking of TFB to DNA. Five femtomoles (≈20,000 cpm) of double-stranded oligonucleotide containing radiolabeled X-UP (lanes 1–4) or X-DO (lanes 5–8) were incubated with 20 ng of the indicated proteins and irradiated as described in Materials and Methods prior to electrophoresis on an SDS/12% polyacrylamide gel. The position of TFB crosslinked to the X-UP-containing oligonucleotide probe is indicated by an arrow; the faint signal below TFB corresponds to a proteolytic fragment of TFB, as confirmed by Western blotting (data not shown).