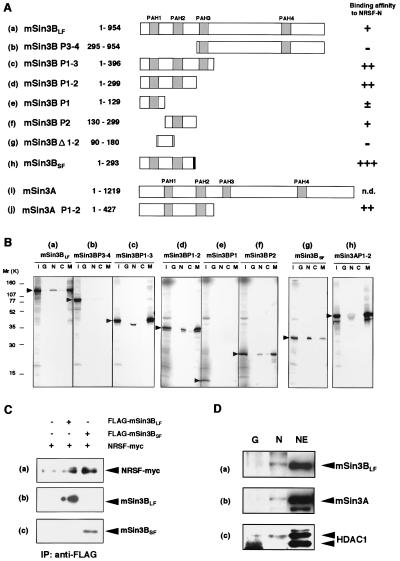
Figure 3.
NRSF-N forms a complex with mSin3 and HDAC in mammalian cells. (A) Regions of mSin3 involved in the interaction with NRSF-N. Relative strengths of NRSF-N and mSin3 interactions were assessed by quantification of the autoradiograms and scored from background level (−) to strong interaction (+++); n.d., not determined. PAH domains and a unique region in mSin3BSF are distinguished by hatched and shaded boxes, respectively. Numbers correspond to amino acid positions in the protein. (B) Specific binding of NRSF-N and mSin3 in vitro. GST-NRSF fusion proteins, i.e., GST-NRSF-N (N), GST-NRSF-C (C), GST alone (G), or GST-MAD (M), were incubated with 35S-labeled FLAG-tagged mSin3 deletion constructs. Specifically bound mSin3 subforms then were resolved on an SDS/PAGE gel and detected by autoradiography. An aliquot comprising one-fifth of the total input protein was loaded in lane I as a control. (C) Interaction of NRSFMyc and mSin3BFLAG in NIH 3T3 cells. Expression plasmids encoding Myc-tagged NRSF and the long or short isoform of FLAG-tagged mSin3B were cotransfected into NIH 3T3 cells, and the expressed mSin3BFLAG was immunoprecipitated with anti-FLAG M2 antibody. Proteins that interacted with mSin3BFLAG were resolved by SDS/PAGE and visualized by Western blotting. Shown are fluorograms of NRSF, visualized by using the anti-myc antibody 9E10 (a), and mSin3BLF (b) and mSin3BSF (c), visualized by using the anti-mSin3B antibody A-20. (D) Association of NRSF-N with mSin3A, mSin3B, and HDAC1 in NIH 3T3 cells. GST pull-down experiments show that NRSF-N interacts with mSin3A, mSin3BLF, and HDAC1. Nuclear extracts of NIH 3T3 cells were incubated with either immobilized GST alone (lane G) or GST-NRSF-N fusion protein (lane N), precipitated, and visualized by using anti-mSin3B (A-20) (a), anti-mSin3A (K-20) (b), or anti-HDAC1 (C-19) (c) antibody. Nuclear extracts were loaded into lane NE as a control.