Figure 3.
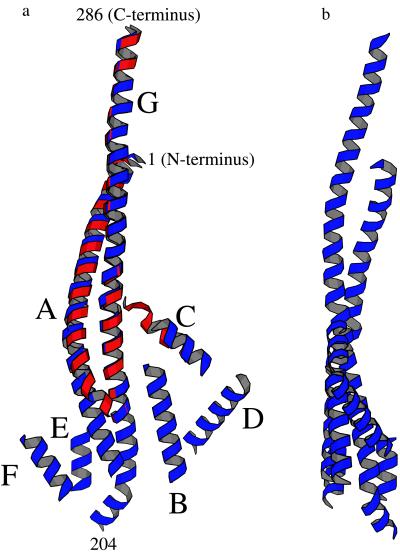
(a) Ribbon drawing showing, in blue, the presumed α-helical segments that appear to constitute part of the γ subunit of ECF1. Each helical segment was placed so as to coincide with a rod of density in the ECF1 electron-density map (Fig. 2a). The direction of view is the same as in Fig. 2a. The approximate positions of selected residues within the α-helical coiled-coil are indicated. The α-helices are labeled A through G. This is primarily for reference, although it does represent one possible path that the polypeptide backbone might follow from one end of the γ subunit to the other. The part of the γ subunit seen in the structure of beef-heart MF1 (10) is superimposed in red. The maximum width, as seen in this view, is about 50 Å and the height about 110 Å. (b) View of the presumed helical region of the γ subunit of ECF1, as in Fig. 3a, rotated 90°.