Abstract
Non-human animals can acquire novel route preferences by following knowledgeable individuals. Such socially learned route preferences can be stably maintained over multiple transmission episodes, sometimes forming long-lived traditions. In humans, preferences for familiar routes or heavily used worn trails over unfamiliar ones have been described in various contexts. However, social learning of route preferences has not been experimentally demonstrated in humans. Here, we demonstrate that social learning and tradition influence route choice. We led adult male and female participants into a room by one of two routes. Participants followed the demonstrated route choices, and later remembered and preferred this choice even when determinably suboptimal (i.e. longer and not preferred by control participants) or when the choice was indicated as arbitrary (the demonstrator took one route to retrieve a poster that had ostensibly fallen). Moreover, route preferences were stably maintained over multiple transmission episodes. We suggest that simple social learning processes, often neglected in human and primate research, can result in long-lived route preferences that may influence a range of additional behaviour patterns.
Keywords: social learning, human behaviour, cultural transmission, route learning
1. Introduction
Non-human animals will follow knowledgeable or experienced individuals (e.g. bats: Wilkinson 1992; birds: Sonerud et al. 2001), which can lead to the acquisition of novel route preferences (e.g. fish: Laland & Williams 1998, Reader et al. 2003; ants: Franks & Richardson 2006). Such learning by following has been particularly well studied in fish, where it can result in long-lived traditions in the wild that extend across generations (Brown & Laland 2003). In laboratory fish, suboptimal routes can be stably transmitted across multiple transmission episodes (Laland & Williams 1998). These routes are suboptimal in the sense that individuals taking this route swim further and receive less food than if they took the alternative route. However, deviating from such a route may involve a cost, if discovery of the alternative involves leaving the safety of the group (Day et al. 2001). Thus, suboptimal traditions may persist owing to a balance between the costs and benefits of maintaining a tradition versus individual discovery (Galef 2003).
In humans, both past experience and the actions of other individuals influence route choice. For example, humans prefer familiar over novel routes (Benthorn & Frantzich 1999), follow others in emergency situations (Helbing et al. 2000) and tend to take heavily used worn paths rather than walking on untouched ground (Helbing et al. 1997). Experiments on crowd behaviour show that a small informed minority can direct the movement of a group of naive individuals without explicit signalling. Furthermore, where the informed individuals have differing direction preferences, the majority preference tends to dictate group direction (Dyer et al. in press). Here, we examine whether social influences can lead to acquisition of a particular route preference, and whether traditions of route choices could thus form, as has been demonstrated in non-human animals. An experimenter or an experienced participant (the ‘demonstrator’) led participants by one of two routes, and we determined whether this experience influenced the participants' subsequent behaviour. Follow-up experiments addressed the formation of traditions, the transmission of suboptimal (lengthy) routes and the role of ‘rationality’ in route learning. Human infants show ‘rational imitation’ (Gergely et al. 2002). That is, they imitate an ostensibly suboptimal behaviour pattern (turning on a lamp with the head rather than the hands) when the demonstrator has no observable reason to use this action (she has her hands free) but not when the demonstrator cannot use the optimal action (she has her hands occupied). Here, participants may demonstrate ‘rational social learning’: reasoning that because the experimenter performed an ostensibly suboptimal action (taking a longer route), the action had unknown benefits or was performed in order to promote learning of this route. We attempted to control for this possibility by, in a final experiment, providing an arbitrary reason for the demonstrator's route choice.
2. Material and methods
(a) Participants
Participants were 72 adult volunteers (40 females, 32 males, mean age 22 years, age range 18–43 years), recruited by posters or lecture announcements around the Utrecht University, The Netherlands. Participants gave written informed consent prior to the experiment. Participants in the experimental treatments completed a number of tasks and questionnaires over approximately 75 min. The route learning experiment was conducted while participants moved between rooms to complete the various tasks, and thus they were not aware of this particular experiment. This assumption was supported by an end-of-session questionnaire, where we asked whether the participants thought that the experimenters were observing anything additional to test performance, if so, what. Participants had varied and numerous ideas about possible extra observations, but none concerned the route choice experiment. Participants were debriefed after the experiment.
(b) Procedure
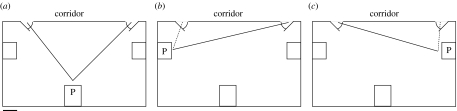
General procedure. An experimenter led single individuals to a corridor and then into a room by one of two routes, each using a different door. Participants were told that they would complete a puzzle task located in another room. While leading participants, the experimenter began to explain the rules of the puzzle. Thus, participants were not overtly instructed to remember, follow or attend to the route. Once participants completed the puzzle, they were asked to return on their own to the starting room to continue the questionnaires. We addressed whether participants followed the experimenter to the puzzle room and whether they maintained a preference for the experimenter-demonstrated route when leaving the room alone to return to the starting room. Demonstrated routes (figure 1) were counterbalanced.
Figure 1.
The experimental room (plan view). (a) Experiments 1–2 (equal routes) and (b,c) experiments 3–4 (long routes). Solid lines, demonstrated routes; dotted lines, the available short route. P, puzzle location. Scale bar, 1 m. Both doors led to the same corridor and were approximately 11 m from the starting room.
We conducted four experiments, with experiment 4 including an additional control condition. Experiment 1 (N=16) used routes of similar length with all doors closed. Experiment 2 used the same set-up as experiment 1, but involved ‘transmission chains’ where participants acted as the demonstrator for the next individual in the chain. The first participant was led into the room by the experimenter as usual and asked to return with the next participant. We conducted four chains, each of 4–6 individuals (including the experimenter) and involving 16 participants in total. Experiment 3 (N=13) adopted an ‘unequal-routes’ design, with the task placed asymmetrically near one exit (‘short route’, 2 m), 7–8 m from the other exit (‘long route’). Participants were led by the long route. The experimenter closed the demonstrated long-route door, walked to the open short-route door and closed it with ‘I will just close the other door’. Thus, the short-route door was verbally and manually marked, and the participants could observe that it led to the corridor. Experiment 4 (N=27, including control participants) repeated experiment 3, but provided an arbitrary reason for the experimenter's long-route choice (a fallen poster to be recovered).
3. Results and discussion
In all four experiments, all participants followed their demonstrator's route into the room. We expected this strong following tendency, given that the participants were unfamiliar with the route, and that entering via the alternative route would require separation from the demonstrator.
(a) Experiment 1
When leaving the room alone, all 16 participants left by the previously demonstrated route, evidence consistent with them having learned this route (Χ2=16.00, p<0.0001). However, this finding does not address whether this learning by following could lead to stable repeated person-to-person information transmission, a possibility addressed in experiment 2.
(b) Experiment 2
In all four transmission chains, a preference for the demonstrated route was acquired and transmitted with perfect fidelity along the chains (binomial test, p<0.0001). This suggests that traditions of route choice can follow from the initial route choice of a single individual. However, in experiments 1 and 2, the arbitrary nature of the route choice, combined with the alternative route requiring exit by a never-opened door, may make use of the unfamiliar, undemonstrated route unlikely. Experiments 3 and 4 addressed this issue.
(c) Experiment 3
All 13 participants left the room by the previously demonstrated long route (Χ2=13.00, p<0.001). Thus, the preference for the demonstrated route was maintained even if it was longer than the alternative route, attention was drawn to the alternative route, and it could be observed to lead to the corridor. However, it is possible that the participants reasoned that the experimenter took the longer route for some unknown reason, and thus it was rational to copy the route preference of the experimenter. Experiment 4 addressed this possibility.
(d) Experiment 4
Out of 18 participants, 17 maintained the long-route choice (Χ2=14.32, p<0.001), providing no evidence that their route preference was maintained because they reasoned that it had unknown benefits. A control condition, where participants were placed at the task sites and asked which route they would take, confirmed that participants could distinguish route lengths. Without demonstration, eight out of the nine control participants preferred the short route. Typical reasons given for this preference were that it was ‘shorter’ or ‘faster’.
4. General discussion
We suggest that the simplest explanation for our findings is that participants, through a propensity to follow others, were exposed to, and thus familiarized with, a route that they subsequently continued to prefer over an unfamiliar route. Other explanations are possible, such as a reluctance to violate social norms, although similar findings in a range of non-human animals (Brown & Laland 2003; Franks & Richardson 2006) remove the need to invoke such explanations. Physical trail formation, such as worn grass in humans (Helbing et al. 1997) or odour trails in rats (Galef 2003), could potentially strengthen the long-term stability of learned route preferences.
Whatever the cognitive mechanism, the formation of stable learned route preferences, as demonstrated here, has various consequences. For example, humans prefer familiar over unfamiliar escape routes, even when the former are longer (Benthorn & Frantzich 1999), and a propensity to follow provides suboptimal escape in emergencies (Helbing et al. 2000). Tendencies to learn routes from others will accentuate such effects. More generally, route learning by following will result in exposure to a particular subhabitat and a set of environmental contingencies, and a suite of individual and social learning about these contingencies can ensue. For example, individuals may individually or socially acquire complex food processing techniques to deal with the resources located in a particular subhabitat (e.g. Humle & Matsuzawa 2002). The complexity of these knock-on effects will depend upon the cognitive capacities of the species involved. Intergroup differences in multiple, complex learned behaviours may arise through cognitively simple acts, such as learning by route following, and we suggest that such simple processes have been neglected in analyses of primate and human traditions (see also Laland & Janik 2006; van der Post & Hogeweg in press).
Humans can acquire a variety of behaviour patterns, fears and preferences by social learning in experimental settings (Bandura et al. 1961; Gergely et al. 2002; Addessi et al. 2005; Jones et al. 2007; Olsson & Phelps 2007). A related, and substantial, literature exists on human conformity and social influences on behaviour (e.g. Bond & Smith 1996), including demonstration that exposure to dissent can lead to later non-conformity (Nemeth & Chiles 1988). Such findings have been extended to ‘real-life’ decision making (e.g. Conrad et al. 1992; Salganik et al. 2006; Bentley et al. 2007). Our study adds a new facet to this rich literature and reinforces the recurrent message that cultural transmission need not require derived, specialized neurocognitive mechanisms (Heyes 2001; Olsson & Phelps 2007). Moreover, we provide a paradigm adaptable to virtual computer worlds, which would allow manipulation of such variables as group composition, the ease of individual discovery, and conflicts between information sources. For example, our paradigm would allow a test and extension of recent theoretical models which suggest that grouping combined with individual trial-and-error learning may result in cumulative increases in foraging efficacy over cultural generations (van der Post & Hogeweg in press).
Acknowledgments
The procedures and questionnaires were approved by the Medical Ethics Review Committee of the University Medical Center Utrecht and comply with APA's ethical guidelines and the principles expressed in the Declaration of Helsinki.
We thank L. Mevis and F. van Rijnsoever for their assistance; N. J. Boogert, K. N. Laland, J. van Stekelenburg, U. Toelch and M. Zdebik for their comments; and the Netherlands Organisation for Scientific Research (NWO) Evolution and Behaviour Programme for funding.
References
- Addessi E, Galloway A.T, Visalberghi E, Birch L.L. Specific social influences on the acceptance of novel foods in 2-5-year-old children. Appetite. 2005;45:264–271. doi: 10.1016/j.appet.2005.07.007. doi:10.1016/j.appet.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Bandura A, Ross A, Ross S.A. Transmission of aggression through imitation of aggressive models. J. Abnorm. Soc. Psychol. 1961;63:575–582. doi: 10.1037/h0045925. doi:10.1037/h0045925 [DOI] [PubMed] [Google Scholar]
- Benthorn L, Frantzich H. Fire alarm in a public building: how do people evaluate information and choose an evacuation exit? Fire Mater. 1999;23:311–315. doi:10.1002/(SICI)1099-1018(199911/12)23:6<311::AID-FAM704>3.0.CO;2-J [Google Scholar]
- Bentley R.A, Lipo C.P, Herzog H.A, Hahn M.W. Regular rates of popular culture change reflect random copying. Evol. Hum. Behav. 2007;28:151–158. doi:10.1016/j.evolhumbehav.2006.10.002 [Google Scholar]
- Bond R, Smith P.B. Culture and conformity: a meta-analysis of studies using Asch's (1952b, 1956) line judgment task. Psychol. Bull. 1996;1:111–137. doi:10.1037/0033-2909.119.1.111 [Google Scholar]
- Brown C, Laland K.N. Social learning in fishes: a review. Fish Fish. 2003;4:280–288. doi:10.1046/j.1467-2979.2003.00122.x [Google Scholar]
- Conrad K.M, Flay B.R, Hill D. Why children start smoking cigarettes: predictors of onset. Br. J. Addict. 1992;87:1711–1724. doi: 10.1111/j.1360-0443.1992.tb02684.x. doi:10.1111/j.1360-0443.1992.tb02684.x [DOI] [PubMed] [Google Scholar]
- Day R.L, MacDonald T, Brown C, Laland K.N, Reader S.M. Interactions between shoal size and conformity in guppy social foraging. Anim. Behav. 2001;62:917–925. doi:10.1006/anbe.2001.1820 [Google Scholar]
- Dyer, J. R. G., Ioannou, C. C., Morrell, L. J., Croft, D. P., Couzin, I. D., Waters, D. A. & Krause, J. In press. Consensus decision making in human crowds. Anim. Behav (doi:10.1016/j.anbehav.2007.05.010)
- Franks N.R, Richardson T. Teaching in tandem-running ants. Nature. 2006;439:153. doi: 10.1038/439153a. doi:10.1038/439153a [DOI] [PubMed] [Google Scholar]
- Galef B.G., Jr . Social learning: promoter or inhibitor of innovation? In: Reader S.M, Laland K.N, editors. Animal innovation. Oxford University Press; Oxford, UK: 2003. pp. 137–152. [Google Scholar]
- Gergely G, Bekkering H, Kiraly I. Rational imitation in preverbal infants. Nature. 2002;415:755. doi: 10.1038/415755a. [DOI] [PubMed] [Google Scholar]
- Helbing D, Keltsch J, Molnár P. Modelling the evolution of human trail systems. Nature. 1997;388:47–50. doi: 10.1038/40353. doi:10.1038/40353 [DOI] [PubMed] [Google Scholar]
- Helbing D, Farkas I, Vicsek T. Simulating dynamical features of escape panic. Nature. 2000;407:487–490. doi: 10.1038/35035023. doi:10.1038/35035023 [DOI] [PubMed] [Google Scholar]
- Heyes C. Causes and consequences of imitation. Trends Cogn. Sci. 2001;5:253–261. doi: 10.1016/s1364-6613(00)01661-2. doi:10.1016/S1364-6613(00)01661-2 [DOI] [PubMed] [Google Scholar]
- Humle T, Matsuzawa T. Ant-dipping among the chimpanzees of Bossou, Guinea, and some comparisons with other sites. Am. J. Primatol. 2002;58:133–148. doi: 10.1002/ajp.10055. doi:10.1002/ajp.10055 [DOI] [PubMed] [Google Scholar]
- Jones B.C, DeBruine L.M, Little A.C, Burriss R.P, Feinberg D.R. Social transmission of face preferences among humans. Proc. R. Soc. B. 2007;274:899–903. doi: 10.1098/rspb.2006.0205. doi:10.1098/rspb.2006.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland K.N, Janik V.M. The animal cultures debate. Trends Ecol. Evol. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. doi:10.1016/j.tree.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Laland K.N, Williams K. Social transmission of maladaptive information in the guppy. Behav. Ecol. 1998;9:493–499. doi:10.1093/beheco/9.5.493 [Google Scholar]
- Nemeth C, Chiles C. Modelling courage: the role of dissent in fostering independence. Eur. J. Soc. Psychol. 1988;18:275–280. doi:10.1002/ejsp.2420180306 [Google Scholar]
- Olsson A, Phelps E.A. Social learning of fear. Nat. Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. doi:10.1038/nn1968 [DOI] [PubMed] [Google Scholar]
- Reader S.M, Kendal J.R, Laland K.N. Social learning of foraging sites and escape routes in wild Trinidadian guppies. Anim. Behav. 2003;66:729–739. doi:10.1006/anbe.2003.2252 [Google Scholar]
- Salganik M.J, Dodds P.S, Watts D.J. Experimental study of inequality and unpredictability in an artificial cultural market. Science. 2006;311:854–856. doi: 10.1126/science.1121066. doi:10.1126/science.1121066 [DOI] [PubMed] [Google Scholar]
- Sonerud G.A, Smedshaug C.A, Bråthen Ø. Ignorant hooded crows follow knowledgeable roost-mates to food: support for the information centre hypothesis. Proc. R. Soc. B. 2001;268:827–831. doi: 10.1098/rspb.2001.1586. doi:10.1098/rspb.2001.1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Post, D. J. & Hogeweg, P. In press. Diet traditions and cumulative cultural processes as side-effects of grouping. Anim. Behav (doi:10.1016/j.anbehav.2007.04.021)
- Wilkinson G.S. Information transfer at evening bat colonies. Anim. Behav. 1992;44:501–518. doi:10.1016/0003-3472(92)90059-I [Google Scholar]