Abstract
Antibody diversity is first generated by rearrangement of immunoglobulin (Ig) genes during B cell development in the bone marrow, and later by antigen-driven diversification in germinal centers (GCs). New data in humans and mice now identify specific B cell populations that may have undergone antigen-independent hypermutation outside GCs.
Rearrangement and assembly of B cell antigen receptor genes generates a diverse antibody repertoire in humans and mice. However, the complete range of mechanisms that generate antibody diversity and produce lymphocytes with specific functional and tissue-specific properties remains controversial. Somatic hypermutation (SHM) is a postrearrangement diversification process in which point mutations are introduced into the DNA encoding the V gene through a mechanism mediated by the enzyme activation-induced cytidine deaminase, and which typically occurs in an antigen-dependent manner within GCs (1, 2). Recent studies suggested the existence of a subset of B cells that undergoes SHM in an antigen-independent fashion outside GCs (3), but this viewpoint has remained controversial. Two studies now lend support for this idea. Weller et al. (4) on p. 1331 of this issue identify a subset of B lymphocytes in human infants that undergoes repertoire diversification via antigen-independent V gene SHM. In mice, Shimomura et al. (5) on p. 1343 of this issue describe a phenotypically and functionally unique B cell subset that completes its development in the large intestine and shows evidence of postrearrangement repertoire diversification by SHM. Collectively, these results suggest that the events associated with B cell development in humans and mice may not be far removed from those in other species, such as sheep, in which antigen-independent diversification occurs in the gut-associated lymphoid tissue (GALT).
The “generation of diversity” question
In the 1980s, the structure of the mouse Ig loci and the mechanisms by which the antibody repertoire is diversified were defined (6). Diversity generated through combinatorial joining of Ig gene segments, with the addition of untemplated nucleotides at the joining ends, was calculated to provide binding sites sufficient to accommodate an almost infinite number of possible antigenic determinants (6). This explanation for the generation of a diverse naive antibody repertoire was so seemingly complete that there appeared to be no room or requirement for alternative or additional mechanisms. This view was reinforced by the discovery that the human Ig loci were structured and rearranged in a manner essentially identical to that in mice (6). But exceptions were eventually found, even among animals in which a degree of evolutionary solidarity might be expected. During B cell development in chickens, for example, rearrangement involves a single VH and a single VL gene segment to form an essentially clonal population of cells that is later diversified by gene conversion, a process by which portions of the rearranged V genes are replaced by sequences donated from an array of pseudo–V genes located upstream of the functional V segment. This occurs in a postdevelopment expansion phase in the chicken's bursa, an appendage of the intestine (7). These V segment substitutions occur independently of antigen binding to the B cell receptor (BCR), although gut bacteria provide an antigen-independent proliferative stimulus. Sheep also undergo a process of postrearrangement diversification (Fig. 1). This process occurs in the ileal Peyer's patches located along the large intestine, and involves SHM rather than V gene conversion (8). Finally, rabbits diversify their limited, rearrangement-derived Ig repertoire in the appendix through both gene conversion (as in chickens) and SHM (as in sheep) (9). In each of these examples, postrearrangement diversification occurs in GALT and is thought to be antigen independent. To date, there has been no definitive demonstration of antigen-independent postrearrangement diversification in mice, although mouse B cells undergo postrearrangement V gene replacement in the bone marrow in response to interactions with self-antigen, a process referred to as receptor editing (10).
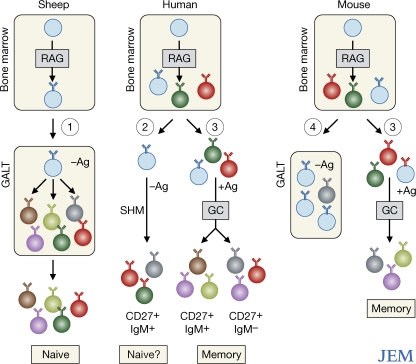
Figure 1.
Comparison of proposed and proven mechanisms of B cell diversification in different species. Early B cell development in all species occurs in the bone marrow, where diversification is generated by recombination activating gene (RAG)–mediated combinatorial joining of the Ig gene segments. In sheep (pathway 1), immature B cells seed the ileal Peyer's patches, where they undergo further repertoire diversification by SHM. This is independent of antigen (Ag) binding to the BCR. These cells then enter the peripheral pool, where they are available for immune responses. An overall similar scheme is followed by rabbits and chickens (see The “generation of diversity” question). The study by Weller et al. (pathway 2; reference 4) proposes an unconventional pathway of diversification in which immature B cells undergo repertoire diversification by SHM in an antigen-independent manner. The location at which this process may occur is uncertain. These B cells appear within the CD27+IgM+ population. Conventional B cell development in humans and mice (pathway 3) relies on antigen-driven diversification in GCs to produce CD27+IgM+ and CD27+IgM− memory B cells. Shimomura et al. (pathway 4) find a distinct subset of B cells that completes its phenotypic maturation in the GALT. There is evidence of SHM among these cells, but it is at a low frequency and of uncertain timing in their development.
Hints of postrearrangement human Ig diversification
The analysis of B cell development and diversification in humans is problematic and, to a degree, has been inferred from analyses of other mammalian species. However, nature often provides material that is every bit as informative as that generated by laboratory geneticists. One example is patients with common variable immune deficiencies (CVIDs). Among this heterogeneous group are individuals with mutations in key genes required for the formation of GCs (11), which, as mentioned, are sites of antigen-driven repertoire diversification mediated by V gene SHM (1, 2). GC B cell survival and entry into the memory compartments is selective and depends on the affinity with which their receptors bind antigen (12), with higher affinity cells expanding preferentially. This antigen-mediated selection results in a distribution of mutations in the V gene segments of memory B cells that is significantly different from the distribution that would be predicted if mutations were inserted at random; changes encoding amino acid replacements are enriched in the antigen-binding regions of the V segments of memory cells, whereas such changes are reduced in the Ig framework regions (2). This skewed distribution is thus an indication of antigen-mediated selection among B cells. Analysis of patients with GC deficiencies has revealed mutations in the genes encoding proteins associated with the provision or receipt of T cell help during immune responses, including CD40, CD40L, SH2D1A, and inducible T cell co-stimulator (11). These patients do not respond appropriately to T cell–dependent (TD) antigens, and are deficient in both serum Ig isotypes other than IgM and B cells expressing isotypes other than IgM and IgD. The deficiency in class switch recombination—an antigen-driven process by which the constant regions of IgM+ antibodies are swapped to form new isotypes—is a reflection of the defective GC formation and the deficiency of T cell help. Curiously, however, some of these patients contain IgM+ B cells that express CD27 (13, 14), a widely used marker of memory B cells in humans. The apparent paradox of “memory” B cells in CVID patients, with their inability to form fully functional GCs, was compounded by the fact that a fraction of the V genes within this IgM+CD27+ memory B cell population showed evidence of SHM. This could be, and has been, interpreted as evidence for a subpopulation of human B cells that behave like those in the ileal Peyer's patches of sheep and diversify their repertoire via a burst of developmentally regulated, antigen-independent SHM (14). It has also been suggested that this mutated IgM+CD27+ B cell compartment is developmentally dedicated to T cell–independent (TI) responses, such as responses to encapsulated bacteria and some of their cell wall constituents (14). This suggestion was based on the localization of these IgM+CD27+ memory B cells in the marginal zone of the spleen, the site at which such TI responses typically develop in rodents (15). Alternatively, mutations in the IgM+CD27+ B cells might result from incomplete and abnormal TD immune responses in these individuals, with B cells exiting the GC after the onset of SHM but before the initiation of class switch recombination (13).
The paper by Weller et al. adds to this debate. The authors studied immunocompetent children younger than 2 years old, before humans acquire the ability to respond to TI antigens (16), making it unlikely that the IgM+CD27+ cells found in these infants are the result of this type of response (4). All of the children in this study had been vaccinated with TD antigens at least once and thus would have generated functional GCs and traditional memory B cells. When the authors compared the distribution of V gene mutations between IgM+CD27+ and IgM−CD27+ (i.e., conventional and isotype-switched) memory B cell populations in these infants, they found that the frequency of mutations (mutations per base pair sequenced) in IgM+CD27+ B cells was similar to that in IgM−CD27+ cells from the same individual, at least when only V genes containing mutations were considered. The two CD27+ B cell populations differed, however, in that the IgM+ population had a higher proportion of V genes carrying no mutations and a greater diversity of clonal V gene rearrangements than IgM− B cells. The authors argue that the restricted Ig repertoire and relative rarity of unmutated sequences (characteristics of antigen-expanded memory B cells) among the IgM−CD27+ B cells is evidence of antigen-driven selection within that population. Although a subset of the IgM+CD27+ population showed evidence of SHM, the broader clonal diversity and relative scarcity of mutated sequences among these cells suggested to the authors that these cells had not undergone antigen-driven diversification, and thus must have been subject to a distinct process of diversification. This is an interesting argument that supports the idea of repertoire diversification by V gene SHM in an antigen-independent manner. However, to accept these data as proof of B cell subpopulations with distinct, if not unique, mechanisms of repertoire diversification, one must accept the assumption that all memory B cells arising from TD immune responses should contain similar patterns of somatic mutation and clonal diversity irrespective of their isotype or time of formation. It is not entirely clear whether or not this assumption is correct, and this remains an outstanding issue in this debate.
Hints of postrearrangement mouse Ig diversification
A common feature of postrearrangement diversification in chickens, sheep, and rabbits is that it occurs in GALT and is not driven by the antigen specificity of the B cells involved (14). Although the vast majority of B lymphocytes in mice and humans develop in the bone marrow according to the “conventional” rules, it remains possible that discrete subsets use distinct developmental pathways, as suggested by the human data from Weller et al. (4) and by B1 cells in mice, which arise in part from fetal precursors and maintain themselves as mature, surface IgM+ cells (17). A second paper in this issue, by Shimomura et al., describes a specific mouse B cell subset that resides in the large intestine (5). This population develops, at least in part, in the intestine where immature IgM+ B cells give rise to a mature IgM+ population (Fig. 1). This transition was assessed through changes in expression of the cell-surface protein CD93 (recognized by the monoclonal antibody AA4.1), which is often used to follow B cell maturation in the spleen (18). The authors show that the development of this intestinal B cell population does not depend on the specificity of the BCR, on signaling through Lyn or Btk, or on help from T cells, suggesting that these cells develop in an antigen-independent fashion. The B cells were, however, dependent on BAFF for their survival, as are all naive, peripheral B cell subsets beyond the most immature stage of development (19). Curiously, and again with some similarities to sheep, a proportion of the V genes among these intestinal B cells was found to be mutated, albeit at a lower frequency than in splenic IgM+ B cells (5). One possible reason for this difference is that, in a manner similar to that described by Weller et al. for the IgM+CD27+ B cell subpopulation in humans (4), these gut B cells may have undergone antigen-independent diversification by SHM, whereas the mature IgM+ B cells in the spleen are the subject of an antigen-dependent process. Although the number of mutations per V gene in intestinal B cells was low, the presence of mutations was not assessed under conditions in which antigen nonresponsiveness is guaranteed, which must be demonstrated to conclusively make this connection. The unique localization of these B cells in the GALT may suggest a specialized function. Indeed, these cells produced IL-12p70 in response to CpG stimulation and responded to intestinal inflammation by dramatically expanding in number during the recovery period after colitis induction. The development of a phenotypically, anatomically, and functionally distinct subset of lymphocytes usually suggests a specialized role in the immune system. However, as with the B1 population, it may take some time to determine the exact function of these intestinal B cells.
Conclusions
Our current understanding of the immunology of lymphocyte subsets, as defined by phenotype and/or location, is that they have evolved to fulfill a particular immunological need. The assumption is that, to some extent, function follows form such that if we understand the developmental behavior of the lymphocytes, we will gain insight into their purpose. Thus, the identification of B cell subsets that apparently develop in distinct fashions relative to the vast majority of B lymphocytes may suggest distinct, evolutionarily conserved functions. The challenges raised by the studies of Weller et al. (4) and Shimomura et al. (5) are to determine with certainty which processes are being used to diversify these populations and which functions they carry out. A unique developmental strategy may indeed reflect a unique function.
References
- 1.McKean, D., K. Huppi, M. Bell, L. Staudt, W. Gerhard, and M. Weigert. 1984. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA. 81:3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuberger, M.S., J.M. Di Noia, R.C. Beale, G.T. Williams, Z. Yang, and C. Rada. 2005. Somatic hypermutation at A.T pairs: polymerase error versus dUTP incorporation. Nat. Rev. Immunol. 5:171–178. [DOI] [PubMed] [Google Scholar]
- 3.Weller, S., M.C. Braun, B.K. Tan, A. Rosenwald, C. Cordier, M.E. Conley, A. Plebani, D.S. Kumararatne, D. Bonnet, O. Tournilhac, et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 104:3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller, S., M. Mamani-Matsuda, C. Picard, C. Cordier, D. Lecoeuche, F. Gauthier, J.-C. Weill, and C.-A. Reynaud. 2008. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+IgD+CD27+ B cell repetoire in infants. J. Exp. Med. 205:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimomura, Y., A. Ogawa, K. Sugimoto, M. Kawada, K. Sugimoto, E. Mizoguchi, H.-N. Shi, S. Pillai, A.K. Bhan, and A. Mizoguchi. 2008. A unique B2 B cell subset in the intestine. J. Exp. Med. 205:1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alt, F.W., T.K. Blackwell, and G.D. Yancopoulos. 1987. Development of the primary antibody repertoire. Science. 238:1079–1087. [DOI] [PubMed] [Google Scholar]
- 7.Reynaud, C.A., B. Bertocci, A. Dahan, and J.C. Weill. 1994. Formation of the chicken B-cell repertoire: ontogenesis, regulation of Ig gene rearrangement, and diversification by gene conversion. Adv. Immunol. 57:353–378. [DOI] [PubMed] [Google Scholar]
- 8.Reynaud, C.A., C. Garcia, W.R. Hein, and J.C. Weill. 1995. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 80:115–125. [DOI] [PubMed] [Google Scholar]
- 9.Becker, R.S., and K.L. Knight. 1990. Somatic diversification of immunoglobulin heavy chain VDJ genes: evidence for somatic gene conversion in rabbits. Cell. 63:987–997. [DOI] [PubMed] [Google Scholar]
- 10.Nemazee, D. 2000. Receptor selection in B and T lymphocytes. Annu. Rev. Immunol. 18:19–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzer, U., and B. Grimbacher. 2006. Common variable immunodeficiency: The power of co-stimulation. Semin. Immunol. 18:337–346. [DOI] [PubMed] [Google Scholar]
- 12.Tarlinton, D. 2006. B-cell memory: are subsets necessary? Nat. Rev. Immunol. 6:785–790. [DOI] [PubMed] [Google Scholar]
- 13.Tangye, S.G., and K.L. Good. 2007. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J. Immunol. 179:13–19. [DOI] [PubMed] [Google Scholar]
- 14.Weill, J.C., S. Weller, and C.A. Reynaud. 2004. A bird's eye view on human B cells. Semin. Immunol. 16:277–281. [DOI] [PubMed] [Google Scholar]
- 15.Martin, F., and J.F. Kearney. 2000. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory.” Immunol. Rev. 175:70–79. [PubMed] [Google Scholar]
- 16.Zandvoort, A., and W. Timens. 2002. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin. Exp. Immunol. 130:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzenberg, L.A. 2000. B-1 cells: the lineage question revisited. Immunol. Rev. 175:9–22. [PubMed] [Google Scholar]
- 18.Allman, D., R.C. Lindsley, W. DeMuth, K. Rudd, S.A. Shinton, and R.R. Hardy. 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167:6834–6840. [DOI] [PubMed] [Google Scholar]
- 19.Schiemann, B., J.L. Gommerman, K. Vora, T.G. Cachero, S. Shulga-Morskaya, M. Dobles, E. Frew, and M.L. Scott. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 293:2111–2114. [DOI] [PubMed] [Google Scholar]