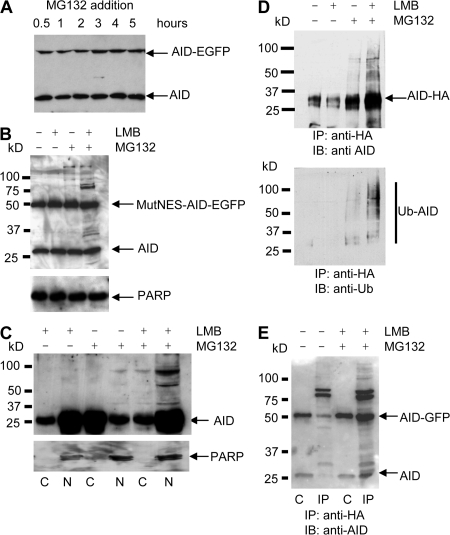
Figure 4.
Ubiquitination of AID and AID-EGFP requires both nuclear localization and proteasome inhibition. (A) AID-EGFP knocked-in BL2 cells, expressing both AID and AID-EGFP under its endogenous promoter, were incubated with MG132 to inhibit proteasome activity. Cell lysates from the indicated time points were analyzed by immunoblotting using anti-AID monoclonal antibodies. (B) BL2 cells expressing both endogenous AID and MutNES-AID-EGFP were incubated for 5 h with LMB and/or MG132, as indicated. AID status was analyzed as described in A. Poly(ADP-ribose) polymerase (PARP; 116 kD) was used as loading control. (C) BL2 cells were incubated with LMB, MG132, or both, as indicated. For each condition, cytoplasmic (C) and nuclear (N) protein extracts were prepared as described in Materials and methods and analyzed by immunoblotting, with monoclonal anti-AID antibodies. Anti-PARP antibody was used as a nuclear protein control. (D) A BL2 cell line expressing WT-AID-HA was incubated with LMB or MG132, or both, for 5 h. After treatment, cell lysates were denatured and immunoprecipitated with agarose-conjugated anti-HA antibodies before SDS-PAGE separation and probing with anti-AID (top) or antiubiquitin antibodies (bottom). MW markers and migration positions of AID-HA are indicated. (E) AID-EGFP knocked-in BL2 cells were transfected with an HA-tagged ubiquitin-expressing vector and either incubated or not with LMB and MG132 for 5 h. Immunoprecipitation was performed 16 h after transfection, as described in D. Western blot analysis was performed using anti-AID antibodies. Lanes: C, extract before immunoprecipitation (1/10th of total extract); IP, immunoprecipitated proteins.