Abstract
It has been suggested that performing a physical action (enactment) is an optimally effective encoding task, due to the incorporation of motoric information in the episodic memory trace, and later retrieval of that information. The current study contrasts old/new recognition of objects after enactment to a conceptual encoding task of cost estimation. Both encoding tasks yielded high accuracy, and robust differences in brain activity as compared to new objects, but no differences between encoding tasks. These results are not supportive of the idea that encoding by enactment leads to the spontaneous retrieval of motoric information. When participants were asked to discriminate between the two classes of studied objects during a source memory task, perform-encoded objects elicited higher accuracy and different brain activity than cost-encoded objects. The extent and nature of what was retrieved from memory thus depended on its utility for the assigned memory test: object information during the old/new recognition test, but additional information about the encoding task when necessary for a source memory test. Event-related potentials (ERPs) recorded during the two memory tests showed two orthogonal effects during an early (300-800 ms) time window: a differentiation between studied and unstudied objects, and a test-type (retrieval orientation) effect that was equivalent for old and new objects. Later brain activity (800-1300 ms) differentiated perform- from cost-encoded objects, but only during the source memory test, suggesting temporally distinct phases of retrieval.
Keywords: enactment, subject-performed task, levels of processing, source memory, retrieval orientation
Over the last twenty-five years, numerous reports have indicated that performing a bodily action during initial study is an effective way of increasing the likelihood that the item will be remembered later. Enacting a bodily movement (“wave your hand”), pantomiming an action with an imaginary object (“brush your teeth”), and manipulating a real object all lead to better recall and recognition of the action phrases than simply listening to them, a phenomenon known as the enactment effect (Arar, Nilsson, and Molander, 1993; Bäckman and Nilsson, 1985; Cohen, 1981; Engelkamp and Zimmer, 1989; Guttentag and Hunt, 1988; Kormi-Nouri et al., 1994; Nyberg and Nilsson, 1995; Svenson and Nilsson, 1989). Encoding by enactment – which we refer to as one variety of action encoding – is also referred to as a subject-performed task (SPT). In most enactment studies, participants are tested on their memory for the verbal commands, by making old/new recognition judgments about action phrases, writing down the commands they remember (free recall), or recalling the verb when given the noun (cued-recall). The memory advantage thus accrues to the verbal phrases which elicited actions during the study phase, whereas memory for the encoding task itself is not evaluated (we review the smaller number of studies which do test memory for the encoding task in the Discussion).
Enactment is a potent encoding task so that, for instance, recognition accuracy after enactment is nearly perfect for up to 80 items (Engelkamp and Zimmer, 1997; Engelkamp et al., 1993; Knopf, 1991; Knopf and Niedhardt, 1989; Mohr et al., 1989; Norris and West, 1991). Encoding by enactment is also effective for older adults and for neurological populations with memory impairments (Bäckman and Nilsson, 1985; Brustrom and Ober, 1996; Butters et al., 1994; Guttentag and Hunt, 1988; Herlitz et al., 1991; Karlsson et al., 1989; Knopf and Neidhardt, 1989; McAndrews and Milner, 1991; Mimura et al, 1998; Nilsson and Craik, 1990; Norris and West, 1991).
The demonstrated efficacy of action encoding has led to strong claims that it has special properties for enhancing (at least) free recall. Zimmer and colleagues have suggested that “By this mechanism, items pop into a person's mind without active search. These data support the theory that performing actions during study enhances the efficiency of an automatic pop-out mechanism in free recall” (Zimmer et al., 2000, pg. 658). Zimmer (2001) further writes: “Very distinct and unique events attract the hippocampal component, and due to this resonance they pop into conscious memory. I assume that this pop-out mechanism, based on item-specific information, is enhanced by SPT, and I also believe that this supplementary mechanism substantially enhances free recall of performed actions. … In summary, automatic retrieval should have a greater influence on memory for SPTs than memory for VTs [verbal encoding tasks].”
We suggest that the apparent ease of retrieval after action encoding has been exaggerated by comparison to very weak baselines. Memory for enacted items is usually compared to memory for items that were merely read or heard with instructions to remember, with no specific judgment or overt response of any sort required – referred to as a “verbal encoding task”. In and of itself, the advantage of an active encoding task over intentional encoding instructions does not suggest any special properties of action encoding as a memory aid, because similar advantages are observed for a variety of encoding tasks over intentional instructions alone (Eagle and Leiter, 1964; Hyde and Jenkins, 1973; Warrington and Acroyd, 1975). Because enacting a verbal command initially requires comprehension of the command, one can wonder whether action-encoding is simply one variety of a deep encoding task (see Kormi-Nori and Nilsson, 2001 for related discussion). One way that enactment is similar to “deep” conceptual encoding is in its lack of sensitivity to incidental versus intentional encoding instructions. When a semantic orienting task is assigned, fore-knowledge of the upcoming memory test is irrelevant (Craik, 1977; Hyde and Jenkins, 1969, 1973). Similarly, instructional manipulations about whether or not memory will tested do not influence recall after enactment (Watanabe, 2003; Zimmer and Engelkamp, 1999). However, the large majority of enactment studies have used intentional instructions, as we do here.
Surprisingly, the literature to date contains no simple evaluation of the efficacy of action encoding as compared to another encoding task that 1) mandates attention to the to-be-remembered stimuli by requiring a judgment about each one, and 2) requires assessment of conceptual properties that are inherent to the stimulus, but not for the domain of action1. A small number of published studies have included some encoding manipulation other than enactment versus intentional-instruction alone, but these have been designed to assess whether the benefit of the other manipulation is additive with the benefit of enactment (Cohen, 1981; Nilsson and Craik, 1990). Zimmer and Engelkamp (1999) asked participants to judge whether a letter triplet occurred in an action phrase (nonconceptual task), or judge whether the described location was a good one for the action (conceptual task, e.g., “apply the postage stamp in the post office” or “…in the pub”). In both cases, the action phrases were performed after the judgment. Two additional study tasks consisted of the conceptual and nonconceptual tasks alone. For the no-enactment conditions, the conceptual encoding task led to higher recall than the shallow task. Free recall performance after action-plus-conceptual encoding was equivalent to conceptual encoding alone. From these results, one might conclude that the conceptual encoding task did all the work, and that action encoding did not add any additional benefit. After observing similar results, Nilsson and Craik (1990) suggested that “…the benefit of SPTs over verbal commands has something in common with the benefit associated with deep as opposed to shallow encoding… By this line of argument, SPTs are one means by which deep encodings may be achieved” (pg 320). However, it is also possible that the design of Zimmer and Engelkamp's (1999) study was non-optimal for finding a specific benefit of action encoding: the action-encoding conditions required the performance of two encoding tasks (action plus additional deep or shallow task), whereas the non-action encoding conditions required the performance of only a single task (deep or shallow). It is possible that dividing attention between two study tasks diminished the benefit that might be obtained with action encoding alone (see Craik, Govoni, Naveh-Benjamin, and Anderson, 1996; Fernandes and Moscovitch, 2000 for the deleterious effects of divided attention at study).
In the present experiment, the efficacy of enactment for old/new recognition is compared to another encoding task that is cognitively effortful, but has no action component. Participants conduct a single encoding task on each trial. On Perform trials, they are asked to perform a typical action with a real object; on Cost trials, they are asked to verbalize their estimate of the object's cost. Both encoding tasks thus require that the object be identified and involve retrieval of some information about its typical attributes. Both encoding tasks also involve a self-initiated strategy to produce an acceptable response because the actions to be performed are selected by the participant rather than the experimenter, and it is similarly left to the participant to determine the basis of a cost estimate. Given the beneficial properties shared by the two encoding tasks, we predict high levels of recognition accuracy for the objects after both tasks. Comparison of old/new recognition accuracies will, however, allow the first test of whether action encoding is better than a deep, but purely conceptual encoding task.
In addition to the idea that retrieval is especially effortless and automatic after enactment, the quotes below illustrate a second strong claim in this literature -- that motoric information becomes part of the episodic memory trace after action encoding, and that this information is accessed during retrieval, even when the retrieval-phase task is only to recall or recognize verbal cues (see also Bäckman et al., 1986; Engelkamp and Zimmer, 1984,1994 for earlier formulations of this idea). “From the multi-system point of view, VTs [verbal tasks] and SPTs [subject-performed tasks] differ due to the fact that only processing of SPTs involves activation and later reactivation of information in the nonverbal motor system and that the enactment effect is largely due to the use of this system as compared to learning the phrases only verbally in VTs” (Engelkamp and Jahn, 2003, pp. 149-150). “Verbal retrieval following encoding enactment should, therefore, involve motor brain areas” (Nilsson et al., 2000, pg. 2199).
The general idea that memories for episodes consist of qualitatively different bits of information distributed across multiple modality- or domain-specific brain regions is one with wide acceptance among memory researchers (Damasio, 1989; McClelland et al., 1995; Paller, 1997). The proposal that memory for an episode with motor activity should include motor regions of cortex is consistent with this broad framework. However, the quotes above exemplify a stronger claim, namely that the motor component of an action episode is accessed even when such access is not required by the memory test, as in the case of recognition or recall of verbal action commands. It is not universally assumed that all aspects of an episode – even if they were successfully encoded – are always retrieved when one is retrieved. The alternative is that the extent and the nature of what information is retrieved is under some degree of voluntary control, and that people often do not retrieve more than what is necessary for their current goals or assigned task.
Demonstrations of incomplete retrieval come from studies of the misinformation effect in eyewitness testimony. In the basic paradigm, participants view a simulated accident or crime, and then hear misleading information about the event. In standard recall or recognition tests, participants frequently produce or endorse the suggested, but false information. However, if explicitly queried about the source of their memory – whether they actually saw the critical item or only heard about it, participants are much less likely to claim that the suggested item was part of the original event (Lindsay and Johnson, 1989; Zaragoza and Koshmider, 1991; Zaragoza and Lane, 1994). This result suggests that people frequently do not access all of the information in their memory when they are not explicitly asked to do so, and is consistent with other behavioral data indicating that item-level information is retrieved before information about how an item was learned, such as whether it was seen or imagined (Johnson et al., 1994).
It remains possible that encoding by active movement results in spontaneous retrieval of motoric information from memory, even when the assigned memory test does not demand this. In the present study, we compare brain electrical activity during retrieval when participants are instructed only to judge photos of objects as studied or unstudied, and also when they are required to additionally differentiate studied objects as encountered in the Perform versus the Cost encoding tasks. In the latter type of test – a source memory test – we expected that Perform-encoded and Cost-encoded objects would elicit different brain activity. In previous work, we've observed fine-grained differentiation of ERPs recorded during retrieval, depending on the nature of the prior encoding task, and have suggested that some aspects of these retrieval-phase ERPs reflect re-engagement of motor cortex after action encoding (Senkfor, submitted; Senkfor et al., 2002). The new question here is whether a similar difference between Perform- and Cost-encoded objects will emerge during the simple old/new recognition test, when retrieval of the motoric or nonmotoric details of the encoding episode is not requested.
Event-related potentials are sensitive to both successful retrieval of information from episodic memory, and also to how test cues are flexibly processed during memory tests, two useful properties that are briefly reviewed below.
Old/new effects in recognition memory tests
In both simple old/new recognition tests and in source memory tests, hit trials are associated with more positive potentials than correct rejections, beginning as early as 200 ms after stimulus onset and evident across most scalp sites. Because hits also elicit larger positive potentials than either false alarms or misses, this effect is associated with successful retrieval (Rubin et al., 1999; Senkfor and Van Petten, 1998; Van Petten and Senkfor, 1996). Much the same effect is elicited during incidental repetition of stimuli as participants perform some non-episodic task, and is reduced or eliminated after damage to the medial temporal lobe or diencephalon leading to amnesia (Olichney et al, 2000).
When participants are asked to retrieve additional information about a studied stimulus in a source memory test, the early old/new effect is accompanied by an additional, later, old/new effect with a focal distribution over prefrontal cortex (Friedman, Cycowicz, & Bersick, 2005; Johansson et al., 2002; Johnson et al., 1997; Kuo and Van Petten, 2006; Ranganath and Paller, 2000; Senkfor, 2002; Senkfor and Van Petten, 1998; Trott et al., 1997; Van Petten, Senkfor, and Newberg, 2000; Wilding, Doyle, and Rugg, 1995; Wilding and Rugg, 1996). For instance, studied words elicit an early old/new effect relative to new words in all recognition tests, but if participants must additionally determine whether the talker's voice is the same or different as during the original presentation, old items additionally elicit a later prefrontal positivity. Similar results are observed across different varieties of information, including spatial location, modality, color, and temporal order. Because the prefrontal old/new effect begins only ∼700 ms after stimulus onset, it reflects a mnemonic process that is engaged after initial item recognition has occurred, or is at least well underway. The stronger engagement of prefrontal cortex by source memory tests over item memory tests in ERP results is consistent with neuropsychological and hemodynamic imaging results (Dobbins et al., 2002; Glisky et al., 2001; Janowsky et al., 1989; Raye et al., 2000; Rugg et al., 1999).
Source retrieval
In our studies with high levels of item recognition accuracy, the prefrontal old/new effect has been insensitive to whether or not the source information accompanying each item is successfully recovered. Hit/hit trials with correct judgments about both the studied/unstudied status of a stimulus, and whether the source attribute is the same or different as during initial study elicit prefrontal ERPs that are largely identical to hit/miss trials. Successful retrieval of source information is instead reflected in differences at other scalp sites, with a very late onset ∼800 ms after stimulus onset (Senkfor and Van Petten, 1998; Senkfor et al., 2002; Van Petten and Senkfor, 2000). The temporal separation between the early old/new effect and the late differentiation between items associated with accurate versus inaccurate source retrieval confirms reaction time evidence (Johnson et al., 1994) in suggesting that episodic memory retrieval can occur in stages, with initial recognition of a test cue as old preceding recovery of more detailed source-specifying information about how it was learned.
We have examined source memory after action-encoding, and observed that late ERPs (more than 800 ms after stimulus onset) also provide good differentiation among conditions with qualitatively different source attributes. We intermixed four encoding tasks of Perform, Watch the experimenter perform an action, Imagine performing an action, and the Cost estimation task (Senkfor, submitted; Senkfor et al., 2002). During a subsequent source memory test, participants viewed digital photos of the objects and attempted to identify the original encoding task. In the retrieval phase, different sets of conditions clustered together over different regions of the scalp. At frontocentral sites overlying premotor cortex, the three conditions that followed action encoding elicited indistinguishable ERPs, but all were distinct from responses to objects that had undergone cost estimation. This action retrieval effect confirmed the prediction based on non-mnemonic studies indicating a commonality among action execution, imagination, and observation (Grezes and Decety, 2001; Rizzolatti et al., 2002). At sites overlying visual cortical regions, electrical activity instead showed very similar responses to Perform- and Watch-encoded objects, which differed from both Imagine- and Cost-encoded objects. This division tracked the split between episodes involving moving hands and moving objects versus episodes with stationary hands and objects, so that we have considered this posterior effect one of motion retrieval. These prior results suggest that action-encoding leaves a specific signature in memory, and that retrieval of action episodes differs from those with only conceptual encoding. However, because participants were explicitly asked to retrieve information about their prior activities, these data do not reveal whether encoding by enactment results in spontaneous retrieval of action information.
Retrieval orientation
Retrieval orientation refers to the processes applied to a test-phase stimulus in order to generate an internal retrieval cue used to search memory. For instance, if one wanted to remember words that were synonyms of a visual word (the cue) versus words that rhymed with it, one might differentially process the semantics vs. the phonology of the cue. These processes were first discussed in the context of how direct (or explicit) and indirect (implicit) memory tests might differ (Nelson et al., 1987; Roediger et al., 1989), but have recently been extended to differences among conditions in direct memory tests. Rugg and colleagues have shown that ERPs to new items in recognition tests vary depending on whether the participants attempt to remember pictures or words from the study phase. These effects take the form of more negative ERPs for test cues that are physically dissimilar from the sought-after information, and are evident over a broad region of the scalp (Herron and Rugg, 2004; Hornberger et al., 2004; Robb and Rugg, 2002; see also Stenberg et al., 2006). These studies suggest that the retrieval of specific varieties of information from an episode is subject to a fair degree of voluntary control, such that test cues undergo differential processing depending on the specific demands of the memory test.
The source versus item memory instructions used here are also a type of retrieval orientation manipulation. The explicit requirement to retrieve information about the encoding task (Perform or Cost) may elicit attention to different features of the objects as compared to the item memory test where this requirement is absent.
Questions and predictions in the current experiment
The current experiment contrasts item (old/new) and source memory tests for action-encoded (Perform), conceptually-encoded (Cost), and unstudied stimuli. After studying real objects during the learning phases, digital photos of old and new objects are presented during the two memory tests. Some of our predictions follow closely from prior results: that an early old/new effect will be observed in both memory tests, and that Perform vs. Cost old items will show a later differentiation in the source test. New questions are stimulated by the behavioral literature on the enactment effect. First, will Perform-encoded items garner higher recognition accuracy than Cost-encoded items in the item recognition test? If so, this will add further weight to the claim that encoding by enactment is superior to other forms of encoding. Second, will brain activity differ for Perform- and Cost-encoded items during the item memory test, when participants are not asked to retrieve this information? If so, this will suggest that some varieties of source-specifying information are retrieved spontaneously, as predicted by the claim that motor cortex is re-activated during retrieval after action-encoding. Finally, will brain activity show a retrieval orientation difference between the item and source memory tests, evident in comparisons between unstudied items in the two tests? If so, this will extend the generality of previous reports using different materials and designs. In contrast, a null effect might suggest that the demand to retrieve information about one's own activities does not require a specific retrieval orientation, perhaps because this sort of retrieval is always attempted, regardless of instruction.
Methods
Participants
Eight men and eight women, between 18-30 years of age (mean = 21 years) were paid for their participation. All were right-handed and had normal, or corrected to normal, vision and color vision by self report. Data from four additional participants were not analyzed: one did not return for the second session and three were eliminated during the first session due to eye strain.
Stimuli
Six hundred familiar objects or toy versions of familiar objects (e.g. stapler, sword, lawnmower) were presented during the study or test phases. Of the total set of objects, 474 could be manipulated with one hand when performing a typical action (such as a pencil); the other 126 required two hands (such as shuffling a deck of cards, or holding the deck with one hand and dealing with the other). An additional 23 objects were used for practice. All objects were photographed in color and digitized. Color images were presented against a black background during the memory tests.
Electrophysiological methods
The electroencephalogram was recorded via tin electrodes embedded in an elastic cap (Electrocap International). Recording sites were array of 26 equidistant electrodes (Ganis et al., 1996) and the right mastoid. In addition, PfL and PfR electrodes were placed above the nasion (5% of the nasion to inion distance) and 10% of the interaural distance laterally. Vertical eye movements and blinks were monitored via electrodes placed below the right and left eyes. The scalp and vertical EOG electrodes were referenced to the left mastoid during recording, and re-referenced off-line to an average of the left and right mastoids. Horizontal eye movements were monitored via a right to left bipolar montage at the external canthi of the two eyes. The EEG was amplified with half amplitude cutoffs of .01 to 100 Hz, digitized on-line at a sampling rate of 250 Hz. Trials with artifacts due to eye movement or amplifier saturation were rejected prior to averaging. A digital bandpass filter of .01 - 5 Hz was applied to the averaged waveforms.
Procedure
The experiment was conducted across two sessions, each lasting approximately 3.5 hours. The first session consisted of a study phase followed by an item recognition test phase; the second session consisted of an identical study phase (with different objects), followed by a source memory test. This test order was selected in order to avoid possible carry-over effects between the sessions, because both memory tests required a differentiation between studied and unstudied objects, but the source test additionally required that studied objects be distinguished as Perform-encoded or Cost-encoded. Thus, the item memory session was always conducted first, before participants were informed about the source memory aspect of the experiment. The results indicated that item recognition accuracy was unchanged across the two sessions, suggesting that there was no general practice effect across sessions (see also Senkfor & Van Petten, 1998).
During the item study session participants were exposed to 150 objects placed one at a time on a table in front, to the right of, or left of the participant. As each object was presented, participants were instructed via a tape recorder, in random order, to estimate the object's cost or to perform a typical action with it. Half of the objects were judged for Cost, and half were manipulated in the Perform condition. In the Perform condition, the right hand was used to manipulate objects on the participant's right, the left hand for objects placed to the left, and both hands for objects placed in front of the participant. Objects presented to the left or right side were those that could be manipulated with one hand; objects centered in front of the participant were those that required two hands. Participants were given 7 seconds to estimate the Cost or Perform an action at which time a tone signaled the object's removal by the experimenter. Another object was presented to the participant approximately four seconds later. Participants touched the object only in the Perform condition.
The recognition test included digital images of all studied objects (150) intermixed with an equal number of new objects. Images were presented for 300 ms, followed by a blank screen for 2700 ms, followed by a signal to respond. Participants made a verbal response (“Old”/“New”) to each image. Before the actual study phase began, participants received a practice set of 12 objects for Perform/Cost encoding tasks; they were informed of the upcoming recognition test but were not told about the relevance of the two encoding tasks. Participants also received a practice set of 23 images just before the item recognition phase of the experiment.
The second session employed the same study tasks as the first. Participants were informed that they would be tested not only for their memory of the object but also the accompanying encoding task. The recognition phase was also similar in that digital images of all the studied objects were included plus an equal number of new objects. When signaled, participants reported verbally whether the object was “New”, “Perform”, or “Cost”, the latter two implying that they considered it an old object. Typically, there was a 10-15 minute delay between the end of the study phase and the beginning of the test phase.
Sixteen different stimulus lists were constructed from four sets of 150 items to counterbalance encoding tasks (Perform/Cost), encoding hands (right/left), old/new objects, and item/source memory tests2. Both the item and source memory tests included 150 new objects, 75 studied in the Perform task, and 75 studied in the Cost task.
Results
The results are presented in four sections describing memory performance, ERPs at test elicited by studied versus unstudied objects, ERPs at test elicited by Perform versus Cost encoded objects, and finally a comparison of ERPs elicited during the item versus source memory tests.
Memory performance
Item memory accuracy
Identification of objects as studied or unstudied (hits and correct rejections) was quite accurate overall and equivalent for the item and source memory tests (see Table 1). An ANOVA using test-type (item vs. source) and encoding condition (Old-Perform, Old-Cost, New) as factors revealed no main effect or interaction involving test type (F's < 2.0), nor a main effect of encoding task (F < 1.0). For both the item and source tests, separate ANOVAs on hit rates alone revealed that objects from the two encoding tasks were remembered equally well (F's < 1.0). Given the generally high accuracy levels, the results were examined for ceiling effects that may have precluded observing a genuine benefit of Perform over Cost encoding. The 95% confidence intervals for accuracy were 92% to 96% for the Perform-encoded objects, and 89.5% to 94.5% for the Cost-encoded objects; the upper limits of these confidence intervals thus did not reach perfect performance. Every participant misclassified some Perform- and some Cost-encoded objects as new, which cannot be attributed to forgetting which button to press, as the responses were oral.
Table 1. Mean percent (standard error) accuracy levels for item and source recognition tests.
| Item test | ||
|---|---|---|
| Hit | 93 (0.8) | |
| Perform | 94 (0.9) | |
| Cost | 92 (1.2) | |
| CR | 95 (0.8) | |
| Source test | ||
| Hit | 94 (0.5) | |
| Perform | 94 (0.8) | |
| Cost | 94 (1.0) | |
| *Hit/Hit | 90 (3.0) | |
| Perform | 93 (3.1) | |
| Cost | 87 (3.2) | |
| CR | 96 (0.6) |
Hit/Hit refers to trials with correct source judgments (Hit-item/Hit-source), computed as the number of trials with correct Perform or Cost judgments divided by the number of trials with a correct judgment of “old” (Perform or Cost, regardless of which was correct, but excluding trials incorrectly judged as “new”).
Source memory accuracy
Source accuracy was computed as the probability of a correct “perform” or “cost” judgment for trials in which the object was recognized as old. Source accuracy was somewhat higher (93% versus 87%) for perform-encoded objects than cost-encoded objects (F(1,15) = 15.0, p < .005). The false alarm trials did not suggest a bias for “perform” or “cost” responses (F < 2).
ERPs elicited by studied versus unstudied objects
ERP analyses are based on correct, artifact-free trials : (1) new objects (correct rejections, CR) in both item and source memory tests (mean of 120 and 121 trials per subject, respectively), (2) correct classifications of old objects in the item recognition test (hits, mean of 62 trials for Perform-encoded objects, and 60 trials for Cost-encoded objects), and (3) correct classifications of both the object and its encoding task (hit/hits) in the source memory test (mean of 58 and 56 trials for Perform- and Cost-encoded items, respectively).
The ERPs elicited by correctly identified objects during the item and source tests are shown in Figures 1 and 2. In both memory tests, hits elicited more positive ERPs than CRs between 300 and 1300 ms post-stimulus onset. ANOVAs with factors of Hit/CR and Electrode Sites (28 levels) for mean amplitude 300-1300 ms relative to a 100 prestimulus ms baseline showed significant old/new effects in both memory tests (item test: F(1,15) = 15.8, p =.0001; source test F(1,15) = 9.52 p < .01). In both memory tests, the difference between hits and correct rejections was rather small at prefrontal and frontal scalp sites, and larger over more posterior central, parietal, temporal, and occipital scalp sites, as seen in Figure 3. In the later portion of the epoch (∼700 ms onward), prefrontal sites show a larger old/new effect in the source test than in the item test.
Figure 1.
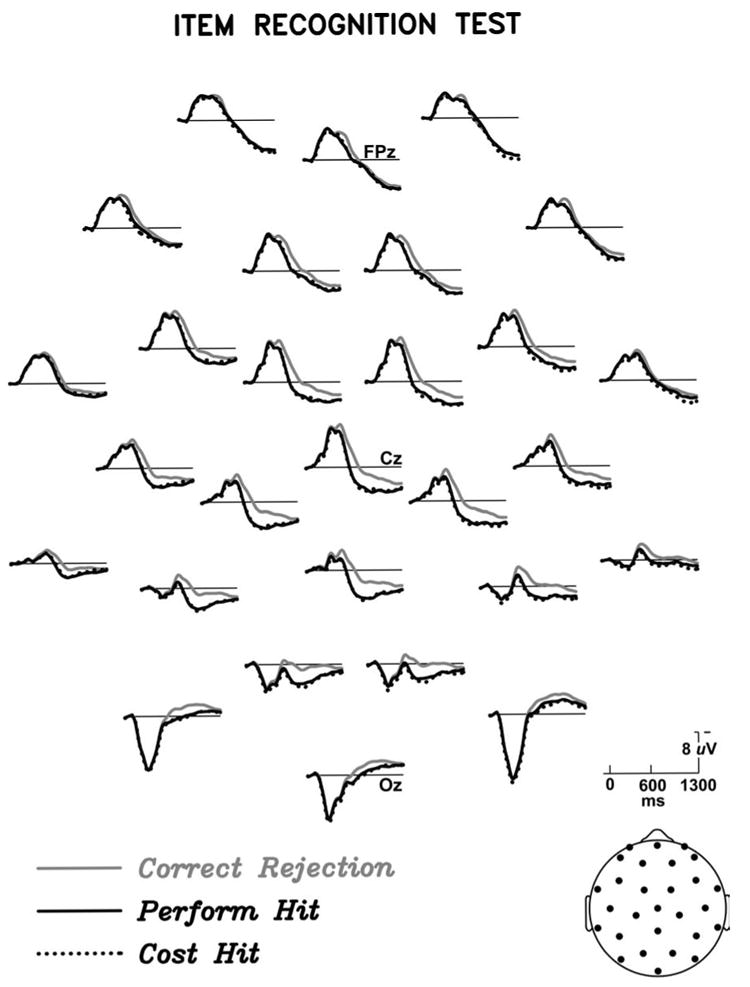
Grand average ERPs for correct trials during the item recognition test, elicited by photographs of objects for which the real three-dimensional object had been studied by performing an action (Perform Hit), studied by estimating the object's cost (Cost Hit), or were new at test (Correct Rejection). The ERPs are plotted in an approximate two-dimensional representation of the scalp electrode placements, with anterior (prefrontal) at the top and posterior (occipital) at the bottom; left in the figure corresponds to left on the scalp. The head icon at lower right also provides a two-dimensional representation of electrode locations. Sites corresponding to those in the 10-20 system are labeled. Positive polarity is plotted down here and in all subsequent figures.
Figure 2.
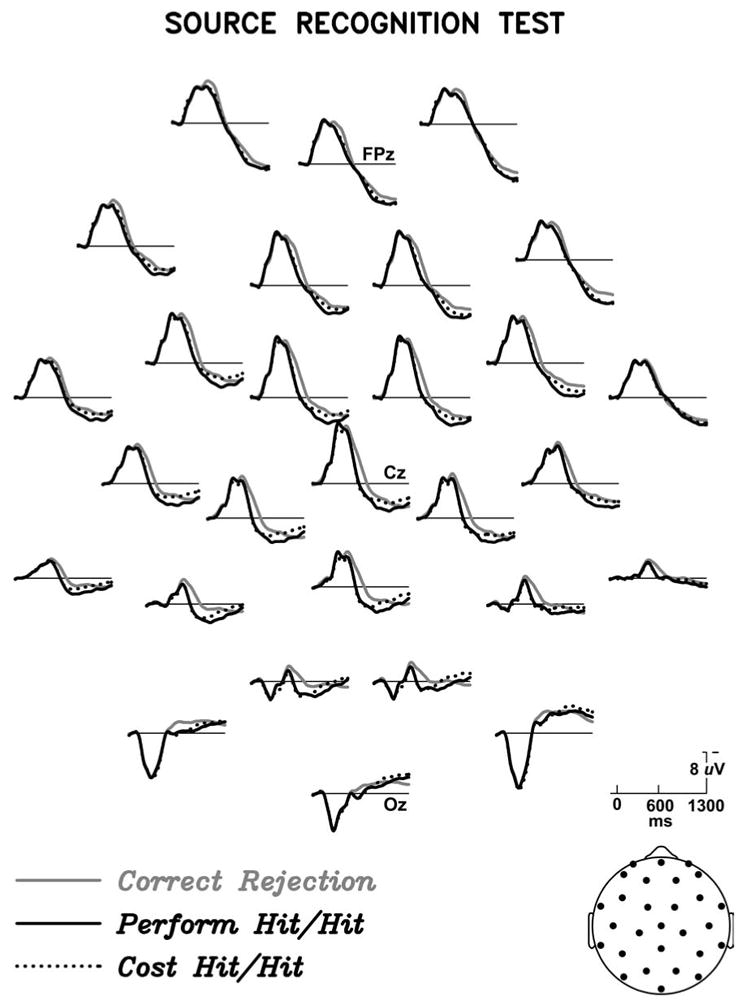
Grand average ERPs from the source recognition test, elicited by photographs of objects for which the real three-dimensional object had been studied by performing an action (Perform Hit/nit), studied by estimating the object's cost (Cost Hit/hit), or were new at test (Correct Rejection). Only correct responses were included in these averages, so that “hit/hit” refers to accurate recognition that an object was studied, and correct recall of the encoding task (performing an action or cost estimation).
Figure 3.
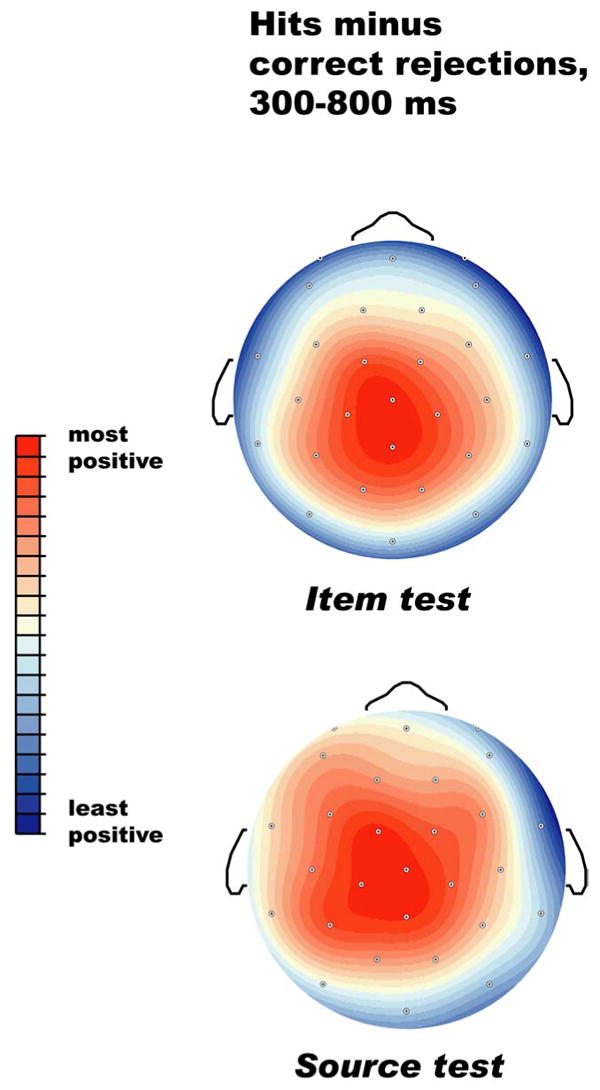
Scalp distribution of the difference between hits (independent of source accuracy) and correct rejections in the item and source memory tests. Both topographic maps have been scaled (normalized) to the range of amplitudes across sites in each of the comparisons, so that red reflects the largest (most positive) difference and blue the smallest (least positive) difference.
ANOVAs with factors of Old/New, Electrodes, and Time window (300-800 vs. 800-1300 ms) show that while the old/new difference in the item test remained constant across the two time windows (F < 2.5, see Figure 4, left column), the old/new effect during the source test showed a second later positivity over prefrontal/frontal sites (Old/New × Timewindow × Electrodes: F(27,405) = 6.69, p < .001, e = .1213; see Figure 4, middle column). This also can be seen in the right column of Figure 4, wherein the old-new effects (difference waves) for the item and source memory tests are overlapped.
Figure 4.
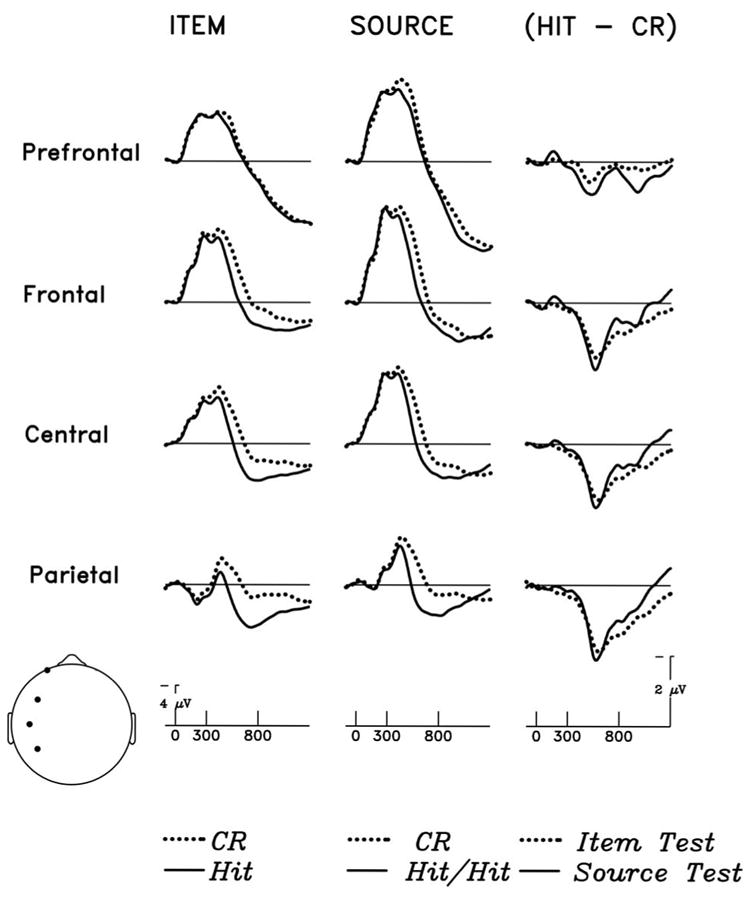
Grand average ERPs elicited by correctly identified studied versus unstudied photographs of objects at four left dorsal sites (depicted in head icon at lower left). During the item test (left column), correctly recognized studied trials (Hits) and correctly identified new trials (Correct rejection: CR) are shown. During source recognition (middle column), Hit/Hit trials are compared to CR trials. Differences between correct studied and new trials from item and source tests are displayed in the right column. The difference waveforms are formed by point-by-point subtractions between two ERP waveforms. For the item test, the difference wave is Hit minus CR; for the source test the difference wave is Hit/Hit minus CR.
Perform versus Cost estimation
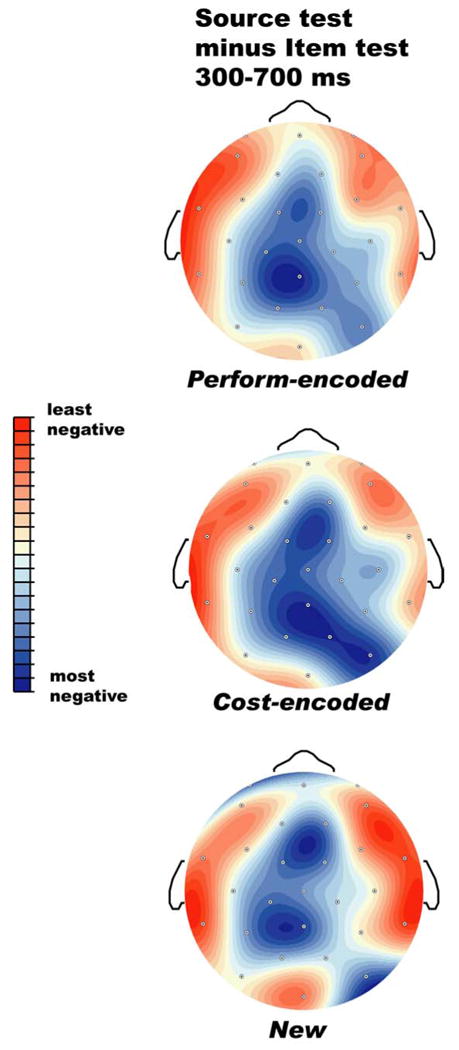
Of greater interest are the comparisons between old objects originally encountered in the two different encoding tasks, given our working hypothesis that action engrams contain information not present after the non-motor task of cost estimation. Figures 1 and 2 show that encoding task (perform vs cost) has no impact on the ERPs during item recognition, but does have an impact on the ERPs during the source test. Specifically, starting around 700-800 ms post stimulus onset, objects from Perform trials elicit a greater positivity than those from Cost trials. Figure 5 provides a closer look at the Perform/Cost difference by showing some scalp sites at a larger scale, and Figure 6 shows the scalp distribution of the difference between Perform- and Cost-encoded objects during the source test.
Figure 5.
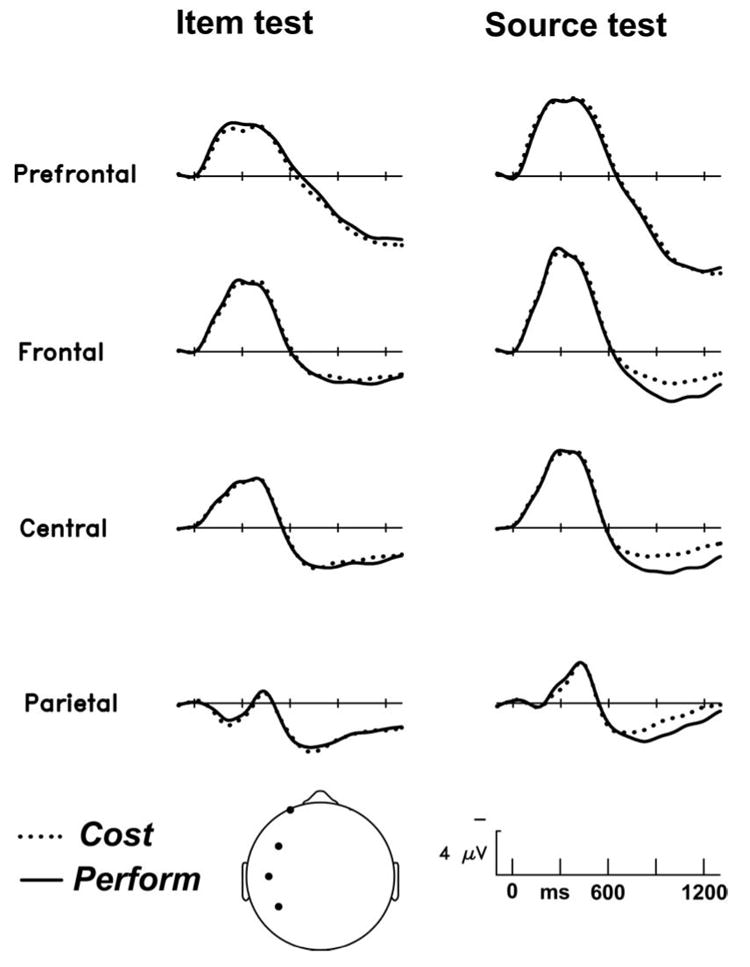
Grand average ERPs elicited by correctly identified photographs of objects initially studied by enactment (Perform) or by Cost estimation, at four left dorsal sites (depicted in head icon, bottom center). Left column shows the old/new (Item) memory test; right column shows the source memory test in which participants judged stimuli as old-perform, old-cost, or new.
Figure 6.
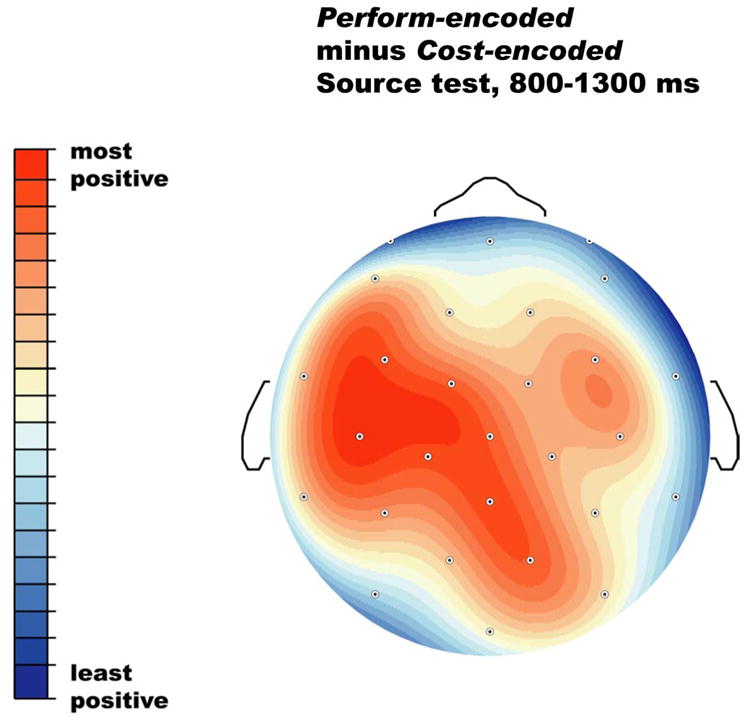
Scalp distribution of the difference between Perform-encoded and Cost-encoded objects during the source memory test. The topographic map has been scaled (normalized) to the range of amplitudes across sites, so that red reflects the largest (most positive) difference and blue the smallest (least positive) difference.
We undertook more detailed analyses of both the temporal and topographic aspects of the ERP as a function of the encoding tasks and memory tests. In these analyses, the old/new effect was divided into two epochs of 300-800 and 800-1300 ms after stimulus onset. In the first set of analyses, we compared “hit” trials – items recognized as old independent of source accuracy in the source test – across prior encoding task (Perform, Cost), test type (Item, Source), and scalp sites (all 28 sites). For the 300-800 ms epoch, there was no significant main effect nor any interactions involving encoding task (F's < 1). For the 800-1300 ms epoch, the main effects of encoding task and test type were nonsignificant (F's < 1.2), but a significant interaction between encoding task and test type was observed (F(1,15) = 5.75, p < .05).
The second set of analyses focused on the consequences of retrieving source information, and thus included only the hit/hit trials (those with correct source judgments) in the source test. For each memory test, the ERPs were analyzed via an ANOVA with encoding task (Perform/Cost) and electrode site (28 levels) as factors for early and late time windows (300-800, 800-1300 ms). During item recognition, no influence of the prior encoding task was observed during either time window (main effect and interactions of Perform/Cost, F's < 1.0). In the source test, the sensitivity of the ERPs to the two encoding tasks was revealed in an interaction of encoding task with time window, which also interacted with scalp location. Encoding task differences occurred during the late time window (Encoding Task × Window: F(1,15) = 7.98, p = .01). The Perform/Cost difference was larger at central, parietal, and occipital sites than prefrontal sites (Encoding Task × Window × Electrode: F(27,405) = 5.02, p = .0005, e = .17).
Follow-up analyses focus on the topography of the Perform/Cost difference in the late time window in the source test, after normalizing to equate overall amplitudes (McCarthy and Wood, 1985). These analyses include 24 lateral scalp sites with three spatial factors reflecting distance from the midline (medial versus dorsal versus far-lateral, MDL), anterior to posterior within the medial, dorsal, and lateral chains (4 levels), and hemisphere (left versus right). This yielded several interactions: Perform/Cost by AP by MDL (F(6,90) = 3.45, p < .05, e = .53), Perform/Cost by AP by hemisphere (F(3,45) = 3.93, p < .05, e =.69), and Perform/Cost by AP by MDL by hemisphere (F(6,90) = 3.58, p < .05, e = .67). These interactions reflect the fact that the Perform/Cost difference was larger over the left than the right, larger at medial and dorsal sites than far lateral sites, and essentially absent at prefrontal sites (see Figure 6).
In sum, images of objects that participants had acted upon during initial exposure elicited more positive ERPs than did those which had originally called for cost estimates, but only when the participants were explicitly requested to remember the original source. Moreover, even in the source test, the Perform/Cost difference occurred substantially later than the initial divergence between old versus new objects. Finally, the content of the source information retrieved - Perform versus Cost - modulated electrical brain activity recorded at posterior, but not prefrontal, scalp sites.
Test-type effects: Item versus Source
The foregoing analyses compared ERPs to objects within each memory test separately. In this section, we evaluate test-type effects for the three conditions of correctly judged objects separately, and describe interactions between encoding condition (perform, cost, new) and test type.
Figure 7 shows three influences of test question. Across all three types of correct judgments, objects presented during the source test elicited more negative ERPs than during item recognition during the first half of the epoch. During the second half of the epoch, objects presented in the source test elicited more positive ERPs than those presented for item recognition at prefrontal scalp sites. At more posterior scalp sites, differential test effects were observed in the late portion of the epoch depending on encoding task.
Figure 7.
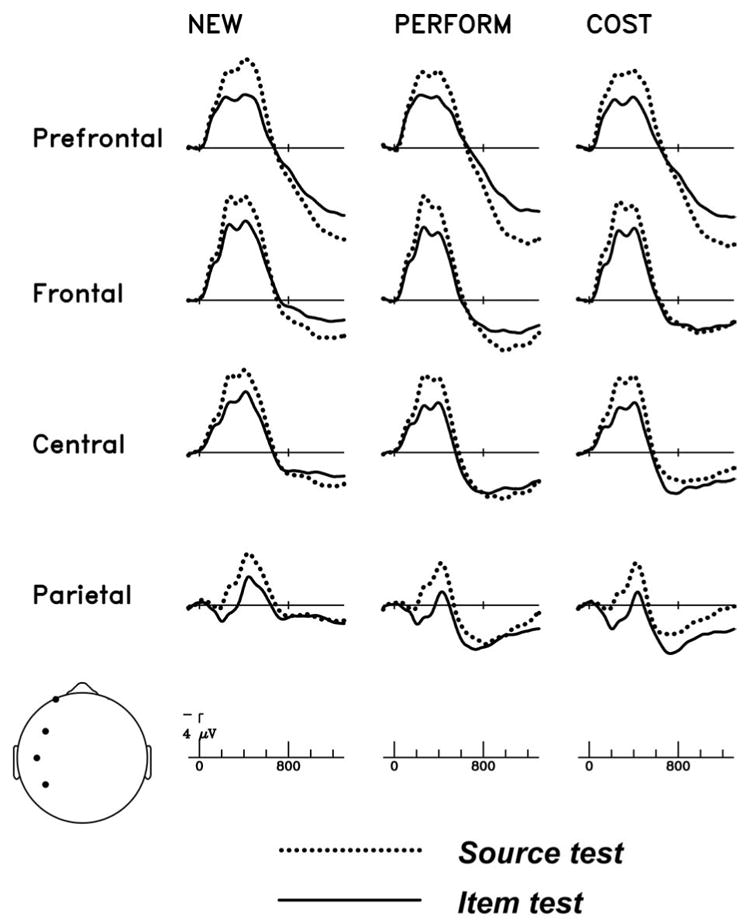
Grand average ERPs elicited at four dorsal scalp sites (locations depicted in head icon at lower left). New trials (Correct Rejection) from the item test are compared to the source test in the left column. Hits from the Perform task in the item test are contrasted with Perform Hit/Hit trials in the source test (middle column), and Hits from the Cost task in the item tests are compared to Cost Hit/Hit trials in the source test (right column).
New items required the same response in the two memory tests. If the nature of the test itself did not influence how items are processed, then new items would elicit the same brain in the item and source tests. In contrast, the left column of Figure 7 shows that simply requesting source information alters the processing of the retrieval cues, resulting in a test-type effect, beginning around 200 ms poststimulus onset. When the ERPs are negative relative to the prestimulus baseline (up until about 700 ms), the source test ERPs are more negative than the item test ERPs. The middle and right columns of Figure 7 show the same test-type effect for Perform- and Cost-encoded items. Figure 8 shows difference waves formed by subtracting ERPs in the item test from those elicited in the source test, and similarly shows that the enhancement of the negative potential was equivalent across Perform-encoded, Cost-encoded, and new stimuli. The scalp distribution of this early test-type effect is shown in Figure 9. The enhanced negativity during the source test was evaluated via ANOVAs taking test-type (item versus source), MDL (3 levels), Anterior/Posterior (4 levels), and Hemisphere as factors for the 300-800 ms latency window. The main effect of test-type was significant for all three types of correct judgments (CR: F(1,15) = 8.24, p < .01; Perform: F(1,15) = 9.20, p < .01; Cost: F(1,15) = 11.0, p < .005). The negative potential was larger closer to the midline (Test × MDL: CR: F(2,30) = 3.74, p < .05, e = .98; Perform: F(2,30) = 4.18, p < .05, e = .90; Cost: F(2,30) = 4.11, p < .05, e=1.0), however, the enhanced negativity in the source test showed no anterior/posterior differences, nor hemispheric asymmetry (see Figure 9).
Figure 8.
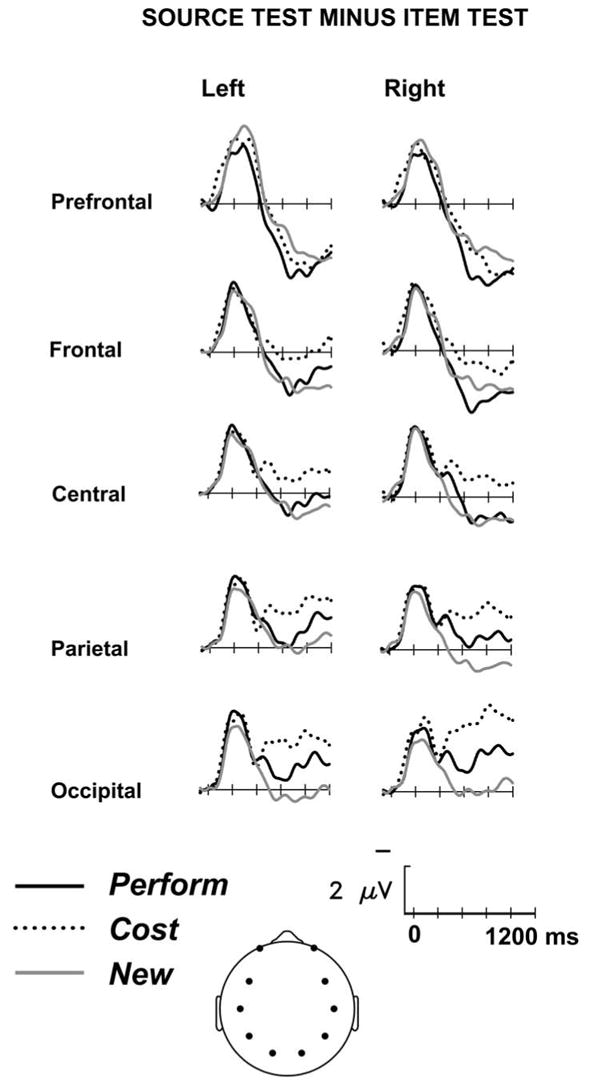
Difference waves showing the influence of test type, for correctly judged objects from the Perform encoding task, the Cost encoding task, and those that were new at test. Shown are left and right dorsal prefrontal, frontal, central, and parietal sites, together with medial occipital sites, as depicted in head icon.
Figure 9.
Scalp distribution of the difference between the source memory test and the item memory test, shown separately for Perform-encoded, Cost-encoded, and unstudied (New) objects. All topographic maps have been scaled (normalized) to the range of amplitudes across sites in each of the comparisons, so that blue reflects the largest (most negative) difference and red the smallest (least negative) difference.
During the second half of the epoch, Figures 7 and 8 show that all three classes of objects presented for source judgments elicited more positive ERPs than those presented for item recognition at prefrontal scalp sites. For studied objects, the enhanced prefrontal positivity during the source test was accompanied by more negative potentials at parietal and occipital sites. In the 800-1300 ms epoch, both the Perform and Cost conditions thus showed test-type by AP interactions (Perform: F(3,45) = 11.4, p < .005, e = .45; Cost: F(3,45) = 6.00, p < .05, e = .41). Correct rejections elicited a different pattern of results. The enhanced prefrontal positivity for objects presented in the source test is visible, but smaller than the test type effect observed for studied objects, and little effect of test type is apparent at the most posterior (parietal and occipital) sites. This pattern of results did not yield a significant test type by AP interaction (F(3,45) = 1.94), nor any other significant interactions involving test type.
Finally, an ANOVA directly comparing the two classes of studied objects (Encoding task, Perform vs. Cost) with factors of Test type, MDL, Anterior/Posterior, and Hemisphere revealed a significant Test by Encoding task interaction (F(1,15) = 6.98, p < .05) in the 800-1300 ms window. Relative to the item test, both classes of old stimuli tended to elicit more positive ERPs over the front of the head and more negative ERPs over the back of the head during the source test. However, the larger frontal positivity during the source test dominated the test-type effect for Perform-encoded objects, whereas the larger posterior negativity dominated the test-type effect for Cost-encoded objects.
In summary, direct comparison of the various types of correct responses (Perform, Cost, CR) across the two memory tests showed both quantitative and qualitative influences of test type. In the early window, the ERPs elicited during the source test were simply larger in amplitude, for both studied and unstudied items. This early influence of test type thus reflects the nature or quantity of information sought in memory, although it occurs during the same latency range as discrimination of old and new objects. In the later epoch, the nature of the test-type effects varied as a function of both the studied status and nature of the prior encoding task, suggesting that these late effects reflect both the success and content of source retrieval.
Discussion
The experiment was designed to investigate three general issues: 1) the efficacy of enactment versus a conceptual but nonmotoric encoding task for promoting successful retrieval, 2) how the attempt to retrieve item versus source information from memory is reflected in brain activity, exemplified here by episodic recognition of objects versus the encoding task in which they were studied, and 3) the degree to which source information is retrieved spontaneously during old/new recognition. These issues are strongly interrelated in the current study, but are discussed in turn.
The efficacy of enactment versus conceptual encoding
The present study includes the first direct comparison of item recognition accuracies after action encoding versus a nonmotoric but conceptually demanding encoding task. Participants were very accurate at recognizing objects encountered during both the cost estimate and the motor tasks. Even with recognition lists of 300 images, 94% of the objects were correctly classified as studied or new. This high accuracy rate confirms the findings of the studies reviewed in the Introduction: action is a very effective encoding aid4. However, our finding of equally high item recognition accuracy after Cost encoding suggests that there is no special memory advantage due to active movement during study. It is possible to maintain that Items encoded by enactment “automatically pop-out into memory” as suggested by Zimmer et al. (2000), but not that this phenomenon is unique following enactment.
Instead, the efficacy of both encoding tasks can more readily be attributed to the features they share. Both tasks require a degree of self-initiation in that participants must select the particular action to be performed, or the best basis for a cost estimate themselves. Recognition performance after both encoding tasks may benefit from some of the same factors that have been invoked to explain the generation effect – the observation that words, facts, numbers, and pictures are more likely to be remembered if the participant had generated the item overtly as opposed to merely seeing or hearing it on initial exposure (deWinstanley, 1995; Gardiner & Rowley, 1984; Peynircioglu, 1989; Slamecka & Graf, 1978). The current encoding tasks differ from the typical generation paradigm in that participants did not, of course, create the objects during the study phase. However, one factor invoked to explain the generation effect is that generation acts to differentiate studied items from one another and thus create more distinctive memory traces (Begg, Snider, Foley, & Goddard, 1989; Mulligan & Duke, 2002). Both the Perform and Cost encoding tasks are likely to serve this function well; participants employed a large number of different actions within the Perform task, and offered many different cost estimates across objects as well.
Both encoding tasks also yielded high source memory accuracy, averaging 90%. Considering the large number of stimuli to be remembered, this level of accuracy is substantially better than previous studies in which the source discrimination based on only one perceptual attribute of the stimulus (such as voice, spatial location, or color; Senkfor and Van Petten, 1998; Van Petten et al, 2000; Kuo & Van Petten, 2006). Despite a number of quantitative similarities (such as the high degree of self-initiation), the Perform and Cost conditions used here were designed to be maximally distinct from one another in qualitative properties, so that multiple attributes of the encoding episode could provide source-differentiating information, including engagement of the motor system, tactile and proprioceptive feedback from handling the object, presence versus absence of visual motion, and the cognitive operations engaged by cost estimation but not action (which could include comparisons to other objects, retrieval of autobiographical memories for purchasing a similar object, etc.). These differentiating attributes arise from the generative nature of both encoding tasks, rather than being intrinsic to the stimuli (i.e., the motor activity, visual motion, somatosensory input, and cognitive operations are all created by the participant). Superior discriminability of self-generated over perceptual sources has also been observed in other studies (e.g. Hashtroudi et al, 1989; Leynes et al., 2005; Senkfor, 2002; Senkfor et al., 2002).
Unlike item recognition performance, memory for source did receive a small (6%) but reliable benefit from action (Perform) over non-action (Cost) encoding. Only a handful of previous studies have investigated source attributions for self-performed actions as compared to other encoding tasks. Cohen and Faulkner (1989) reported 1% higher accuracy for performed as compared to observed actions, and a 14% advantage as compared to imagined actions, but without statistical comparisons. In our previous study, we found significantly higher source accuracy in the Perform condition as compared to observed actions, imagined actions, and the Cost condition (5%, 11%, and 14%, respectively, Senkfor et al., 2002). Hornstein and Mulligan (2004) found a nonsignificant 2% source accuracy advantage for performed as compared to observed actions in a standard comparison, and a reversed effect when participants observed themselves in a mirror while performing. Overall, extant data suggests that (under standard conditions) the source of a self-performed action is more accurately remembered than other sources, but also that the magnitude of this advantage depends on the comparison condition. Self-performed actions are likely to create multiple distinctive tags in memory – more than many encoding tasks – but these may overlap considerably with observed actions. Hornstein and Mulligan's (2004) observation that increasing the visual similarity between performed and observed actions led to reduced source discriminability is consistent with the general idea that source judgments are based on multiple attributes in memory.
Orienting retrieval to item versus source information
Comparisons of brain activity across the two memory tests showed two distinct effects of test type. From about 200 to 700 ms after stimulus onset, ERPs were more negative during the source than item test, independent of whether the items were studied or unstudied. The observation of a test-type effect even for new items indicates that this effect is independent of successful retrieval (of either item or source information), and instead indexes preparation to search memory for source information. Later after stimulus onset (after 700 ms), ERPs showed interactive influences of the studied/unstudied status of the objects and test-type. At prefrontal sites, ERPs were more positive for old objects than new objects in the source test, and this old/new difference was larger than in the item recognition test. Below, we discuss the two test-type effects separately given their different sensitivity to experimental manipulation. Both effects appear similar to observations in other studies, but they have not previously been observed within a single experiment. The current results indicate that the two test-type effects reflect different processes that should not be lumped together as “orientation effects”.
The pure test-type effect in the 200-700 ms epoch is best labeled a true “retrieval orientation” effect as it was insensitive to the studied or unstudied nature of the stimuli, and only sensitive to instruction. The pure test-type effect is broadly similar to the retrieval orientation effects observed in other laboratories for unstudied stimuli, evident as more negative potentials when participants are given retrieval cues that are physically dissimilar to what they have studied. For instance, more negative ERP are elicited when pictures were studied but object names are used as test cues, or vice versa (Herron and Rugg, 2004; Hornberger et al., 2004; Hornberger, Rugg, and Henson, 2006; Johnson and Rugg, 2006; Robb and Rugg, 2002; Stenberg et al., 2006). In a general sense, these negative potentials might reflect the processes required to convert a physical test cue into an internal format more closely aligned with the information sought in memory. If this interpretation is correct, one would expect the scalp topography and wave-shape of retrieval orientation effects to vary somewhat depending on the nature of the initial format (the physical cue) and the target format (the internal retrieval cue), as observed in some of these prior studies using words and pictures (Hornberger et al., 2004; Stenberg et al., 2006). By this perspective, there is no single “retrieval orientation effect”, but rather a set of processes that help to align test cues with stored information. It is thus worth contemplating the specific demands of the current source retrieval test and the specific nature of the retrieval orientation effect in the ERP.
As a starting point, it is relevant to note that even the item recognition test elicited a fairly large negative potential in the 200-700 ms latency range, and that the test-type effect appears to be a modulation of this basic potential. We thus assume that the source memory test was more demanding of some process that also occurred in the item memory test. Large early negative potentials have been a consistent observation in our studies using color photographs of real objects (Senkfor, submitted; Senkfor et al., 2002), but appear to be much less evident in other memory studies using only images at both study and test, and study tasks that do not mandate consideration of three-dimensional object properties (e.g., Hornberger et al., 2004; Johanssen et al., 2002; Kazmerski and Friedman, 1997; Kuo and Van Petten, 2006; Van Petten et al., 2000). In our current and previous studies with objects, the photographic images are visually similar, but not identical to the objects themselves, given the differences between a two-dimensional image and a three-dimensional object. The early negative potential may thus reflect the processes required to mentally translate from a 2D image to a 3D object representation. Schendan and Kutas (2002, 2003) report an “N350” potential that appears similar to the early negative potential here, elicited when participants attempt to identify objects from 2D images. The N350 is enhanced when the mapping from image to object grows more difficult, as when objects are depicted in unusual viewpoints that may require mental rotation to understand their 3D properties.
The hypothesis that the early negative potential reflects extraction of object attributes from a 2D image suggests that the enhancement of this potential during the source memory test reflects greater attention to object properties that are more relevant in the source test, including the 3D properties that are critical for motor interaction with an object. Under this account, the test-type effect reflects an initial stage of transforming the physical retrieval cue – an image – into a format more suitable for a subsequent search of memory, by deriving properties that may (or may not) have been the basis for an action or a cost estimate earlier. Although this retrieval orientation effect occurred during the same latency range as the differentiation between studied and unstudied objects, it was equivalent in magnitude for old and new items. The old/new and retrieval orientation effects in the early latency window were orthogonal and additive. This latter observation is somewhat surprising when one considers that only the old items required source retrieval. The overlapping latency ranges of the old/new and test-type effects suggest that item recognition and preparation to attempt source retrieval may proceed independently rather than in serial order.
A substantial difference between the current study and those from the Rugg group is that we observed identical retrieval orientation effects for studied and unstudied stimuli. After failing to obtain such an effect for studied items, Hornberger et al. (2004) concluded that such effects “were apparent only when retrieval was unsuccessful, as in the case of new items” (pg. 1205). This conclusion is, of course, contrary to the proposal from the same group that such effects reflect processing of retrieval cues “in order to optimize compatibility with targeted memory representations” (Hornberger et al., 2004, pg. 1196), a process that should be independent of retrieval success. Other papers from the same laboratory have simply not included analyses of studied items (Herron and Rugg, 2003; Johnson and Rugg, 2006; Robb and Rugg, 2002). The comparison between physically similar and dissimilar test cues in the picture/word studies may be a suboptimal procedure for isolating cue processing per se, because physically similar cues are likely to elicit stronger matches with memory traces and higher confidence in recognition judgments, thus confounding retrieval success and retrieval orientation for studied items. In the current study, we attribute the observation of a retrieval orientation effect for studied objects to two properties of our paradigm: 1) that recognition of studied objects was equally successful in the item and source tests, and 2) that the test cues contained no information to bias the source judgment in favor of Perform or Cost.
The second test-type effect observed here consisted of a larger prefrontal positivity in the source test than the item test, particularly for studied objects; this test-type effect was most evident in the 800-1300 ms range, well after the differentiation between studied and unstudied objects. During the late latency range of 800-1300 ms, ERPs elicited by new objects showed only small, and statistically nonsignificant differences between the item and source memory tests. The greater impact of test type on hits than correct rejections resulted in a larger old/new difference at prefrontal sites during the source than item test, albeit smaller than in our other work using perceptual source attributes of voice, location, and color (Senkfor & Van Petten, 1998; Van Petten et al., 2000; Kuo & Van Petten, 2006). Late prefrontal effects of test-type are thus different in nature than other test-type effects that influence both studied and unstudied items (and for clarity we suggest that only the latter should be called “retrieval orientation” effects). We have suggested that the late prefrontal engagement reflects the actual attempt to recover source information from memory, after an item has been recognized as old (Senkfor and Van Petten, 1998; Van Petten et al., 2000). The current study indicates that this effect also follows after an earlier stage of retrieval orientation, or generating an internal cue to be used during the source retrieval attempt.
Finally, the late epoch shows more posterior interactions of test type and encoding task – differential brain activity for Perform- and Cost-encoded items in the source test, but not the item test. These late potentials were thus sensitive primarily to the successful retrieval of source information, rather than retrieval orientation per se.
Spontaneous versus voluntary retrieval of source information after enactment
The current study addressed a strong claim in the action memory literature -- that memory for items encoded by enactment is accompanied by re-activation of the motor system, even when participants are not asked to recall their actions. Like the accuracy rates, the brain electrical activity of the current study showed no difference between Perform- and Cost-encoded objects during the item recognition test, providing no support for this idea. In contrast, Perform- and Cost-encoded objects did elicit different brain activity when participants were explicitly asked to remember their study-phase activities, as in two previous studies (Senkfor et al., 2002, submitted). Together with the retrieval orientation effect, the contrasting results of Perform/Cost comparison in the two memory tests strongly suggest that the nature and the extent of memory retrieval is under some degree of voluntary control. In particular, the results suggest that retrieval of a multi-faceted episode can stop at a superficial level, when this is adequate for the demands of the task. In the present experiment, accurate responses in the item memory test could be produced by analysis and recognition of the visual features of the objects, without recourse to other attributes that distinguished objects from the two encoding tasks. The late onset of the Perform/Cost difference in the source test ERPs – as compared to the earlier difference between studied and unstudied objects – indicates that source-differentiating attributes were retrieved after recognition of visual object features. The absence of this late difference in the item recognition test indicates that this second retrieval step was optional, and engaged only when task-relevant.
Our conclusion that action attributes from the study phase are not retrieved when not requested stands in apparent contrast to two positron emission tomography (PET) studies reporting motor cortex activity during memory tests for action-encoded items (Nilsson et al., 2000; Nyberg et al., 2001). Several major differences between the designs of the PET studies and the current experiment can account for the differential results, including 1) the nature of the memory test; 2) presence of new items at test; 3) nature of the retrieval cues; and 4) blocking versus intermixing of encoding tasks. In both PET studies, participants heard action commands (e.g., “make a fist”), and acted them out or simply listened. The memory test was associative cued-recall: when presented with a noun, remember the verb from the same sentence. No unstudied nouns were presented. Finally, the memory tests were administered after blocks of study-phase trials with a single encoding task (all enactment or all listening-only). During the memory test after enacted commands, a participant thus knew that a given noun was associated with an action that he or she had performed earlier, and was asked to recall the name of that action (the verb from the action command). Under these conditions, mentally recreating one's prior actions is likely to be an optimal retrieval strategy, and to be reflected in motor cortex activity.
Conclusions
The item recognition test here followed a study phase of randomly intermixed Perform and Cost encoding trials, and demanded only a discrimination between studied and unstudied items. Because object photos were presented as retrieval cues, many possible strategies were viable for solving the memory problem posed by the test. One could recognize an object by matching its visual features or its name to elements of a multimodal episodic memory. Alternatively, one could internally simulate an action or a plausible cost estimate and determine whether either of these formed a match with an episodic trace. Because Perform- and Cost-encoded objects elicited the same brain activity during item recognition, we conclude that the retrieved information did not include the motoric and somatosensory information that differentiated these memories, but only the visual and/or semantic information that differentiated all studied objects from those that were new at test. The contrast between the item test results on the one hand, and the source test results and PET findings on the other hand suggest that the retrieval of motoric information is driven by its utility for the assigned memory test, rather than being automatic in nature.
A more general conclusion is that although multiple elements of a single episode may be stored, they are not always inextricably bound together, so that retrieval can be selective and partial. This conclusion is consistent with eyewitness testimony and other source memory paradigms showing that source information may or may not be retrieved, depending on the exact phrasing of the test question (Lindsay and Johnson, 1989; Rahal et al., 2002; Zaragoza and Koshmider, 1991; Zaragoza and Lane, 1994)
Acknowledgments
We are grateful to Cynthia May for comments on a previous version of the paper. Funding was provided by the National Institute of Aging (AG 14792, AG 08313) and the National Institute for Mental Health (MH 52893).
Footnotes
Some studies have compared enactment to other varieties of action encoding, such as watching the experimenter perform an action, or imagining performing an action (Arar et al., 1993; Cohen and Faulkner, 1989; Hashtroudi et al., 1990; Koriat et al., 1991). These comparisons are important and interesting for a variety of reasons that are outside the scope of the current paper (see Senkfor et al., 2002), but do not speak to the question of how action encoding may differ from purely conceptual encoding.
Objects requiring two hands for action performance were always presented in the center location.
Huynh-Feldt epsilon correction for inhomogeneity of variance. Original degrees of freedom are reported, but the probability levels reflect the correction.
In pilot work, recognition lists of 400 images (200 studied objects, 200 new) did not reduce item recognition accuracy below 94% or show a difference between Perform and Cost encoding. It will be necessary to examine longer retention intervals to find the limits of the efficacy of these two encoding tasks.
References
- 1.Arar L, Nilsson L, Molander B. Enacted and non-enacted encoding of social actions. Scandinavian Journal of Psychology. 1993;34:126–140. [Google Scholar]
- 2.Bäckman L, Nilsson LG. Prerequisites for lack of age differences in memory performance. Experimental Aging Research. 1985;11:67–73. doi: 10.1080/03610738508259282. [DOI] [PubMed] [Google Scholar]
- 3.Bäckman L, Nilsson LG, Chalom D. New evidence on the nature of encoding of action events. Memory and Cognition. 1986;14:339–346. doi: 10.3758/bf03202512. [DOI] [PubMed] [Google Scholar]
- 4.Begg I, Snider A, Foley F, Goddard R. The generation effect is no artifact: Generating makes words distinctive. Journal of Experimental Psychology: Learning, Memory, and Cogition. 1989;15:977–989. [Google Scholar]
- 5.Brustrom JE, Ober BA. Source memory for actions in Alzheimer's Disease. Aging, Neuropsychology, and Cognition. 1996;3:56–66. [Google Scholar]
- 6.Butters MA, Kaszniak AW, Glisky EL, Eslinger PJ, Schacter DL. Recency discrimination deficits in frontal lobe patients. Neuropsychology. 1994;8:343–353. [Google Scholar]
- 7.Cohen G, Faulkner D. Age differences in source forgetting: Effects on reality monitoring and eyewitness testimony. Psychology and Aging. 1989;4:10–17. doi: 10.1037//0882-7974.4.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Cohen R. On the generality of some memory laws. Scandinavian Journal of Psychology. 1981;27:267–272. [Google Scholar]
- 9.Craik FI, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. Journal of Experimental Psychology: General. 1996;125:159–80. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- 10.Craik FIM. Depth of processing in recall and recognition. In: Dornic S, Rabbitt PMA, editors. Attention and Performance VI. Hillsdale, NJ: Erlbaum; 1977. pp. 679–697. [Google Scholar]
- 11.Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- 12.Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 13.DeWinstanley PA. A generation effect can be found during naturalistic learning. Psychonomic Bulletin and Review. 1995;2:538–541. doi: 10.3758/BF03210990. [DOI] [PubMed] [Google Scholar]
- 14.Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 15.Eagle M, Leiter E. Recall and recognition in intentional and incidental learning. Journal of Experimental Psychology. 1964;68:58–63. doi: 10.1037/h0044655. [DOI] [PubMed] [Google Scholar]
- 16.Engelkamp J. Memory for actions. Hove: Psychology Press; 1998. [Google Scholar]
- 17.Engelkamp J, Jahn P. Lexical, conceptual and motor information in memory for action phrases: a multi-system account. Acta Psychologica. 2003;113:147–165. doi: 10.1016/s0001-6918(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 18.Engelkamp J, Zimmer HD. Motor program information as a separate memory unit. Psychological Research. 1984;46:283–289. doi: 10.1007/BF00308889. [DOI] [PubMed] [Google Scholar]
- 19.Engelkamp J, Zimmer HD. Memory for action events: A new field of research. Psychological Research. 1989;51:153–157. doi: 10.1007/BF00309142. [DOI] [PubMed] [Google Scholar]
- 20.Engelkamp J, Zimmer HD. Motor similarity in subject-performed tasks. Psychological Research. 1994;57:47–53. doi: 10.1007/BF00452995. [DOI] [PubMed] [Google Scholar]
- 21.Engelkamp J, Zimmer HD. Sensory factors in memory for subject-performed tasks. Acta Psychologica. 1997;96:43–60. [Google Scholar]
- 22.Engelkamp J, Zimmer HD, Biegelman UE. Bizarreness effects in verbal tasks and subject-performed tasks. European Journal of Cognitive Psychology. 1993;5:393–415. [Google Scholar]
- 23.Engelkamp J, Zimmer HD, Mohr G, Sellen O. Memory of self-performed tasks: Self-performing during recognition. Memory and Cognition. 1994;22:34–39. doi: 10.3758/bf03202759. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes MA, Moscovitch M. Divided attention and memory: Evidence of substantial interference effects at retrieval and encoding. Journal of Experimental Psychology: General. 2000;129:155–176. doi: 10.1037//0096-3445.129.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Friedman D, Cycowicz YM, Bersick M. The late negative episodic memory effect: the effect of recapitulating study details at test. Cognitive Brain Research. 2005;23:185–198. doi: 10.1016/j.cogbrainres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Ganis G, Kutas M, Sereno M. The search for “common sense”: An electrophysiological study of the comprehension of words and pictures in reading. Journal of Cognitive Neuroscience. 1996;8:89–106. doi: 10.1162/jocn.1996.8.2.89. [DOI] [PubMed] [Google Scholar]
- 27.Gardiner JM, Rowley JM. A generation effect with numbers rather than words. Memory and Cognition. 1984;12:443–445. doi: 10.3758/bf03198305. [DOI] [PubMed] [Google Scholar]
- 28.Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: an encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- 29.Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta analysis. Human Brain Mapping. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttentag RE, Hunt RR. Adult age differences in memory for imagined and performed actions. Journal of Gerontology. 1988;43:107–108. doi: 10.1093/geronj/43.4.p107. [DOI] [PubMed] [Google Scholar]
- 31.Hashtroudi S, Johnson MK, Chrosniak LD. Aging and source monitoring. Psychology and Aging. 1989;4:106–112. doi: 10.1037//0882-7974.4.1.106. [DOI] [PubMed] [Google Scholar]
- 32.Hashtroudi S, Johnson MK, Chrosniak LD. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychology and Aging. 1990;4:106–112. doi: 10.1037//0882-7974.5.1.119. [DOI] [PubMed] [Google Scholar]
- 33.Heil M, Rolke B, Engelkamp J, Roesler F, Oezcan M, Hennighausen E. Event-related brain potentials during recognition of ordinary and bizarre action phrases following verbal and subject-performed encoding. European Journal of Cognitive Psychology. 1999;11:261–280. [Google Scholar]
- 34.Herron JE, Rugg MD. Retrieval orientation and the control of recollection. Journal of Cognitive Neuroscience. 2003;15:843–854. doi: 10.1162/089892903322370762. [DOI] [PubMed] [Google Scholar]
- 35.Herlitz A, Adolfsson R, Bäckman L, Nilsson LG. Cue utilization following different forms of encoding in mildly, moderately, and severely demented patients with Alzheimer's Disease. Brain and Cognition. 1991;115:119–130. doi: 10.1016/0278-2626(91)90020-9. [DOI] [PubMed] [Google Scholar]
- 36.Hornberger M, Morcom AM, Rugg MD. Neural correlates of retrieval orientation: Effects of study-test similarity. Journal of Cognitive Neuroscience. 2004;16:1196–1210. doi: 10.1162/0898929041920450. [DOI] [PubMed] [Google Scholar]
- 37.Hornberger M, Rugg MD, Henson RN. ERP correlates of retrieval orientation: direct versus indirect memory tasks. Brain Research. 2006;1071:124–136. doi: 10.1016/j.brainres.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 38.Hornstein SL, Mulligan NW. Memory for actions: Enactment and source memory. Psychonomic Bulletin and Review. 2004;11:367–372. doi: 10.3758/bf03196584. [DOI] [PubMed] [Google Scholar]
- 39.Hyde TS, Jenkins JJ. Differential effects of incidental tasks on the organization of recall of a list of highly associated words. Journal of Experimental Psychology. 1969;82:472–481. [Google Scholar]
- 40.Hyde TS, Jenkins JJ. Recall of words as a function of semantic, graphic, and syntactic orienting tasks. Journal of Verbal Learning and Verbal Behavior. 1973;12:471–480. [Google Scholar]
- 41.Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- 42.Johansson M, Stenberg G, Lindgren M, Rosén I. Memory for perceived and imagined pictures: An event-related potential study. Neuropsychologia. 2002;40:986–1002. doi: 10.1016/s0028-3932(01)00148-8. [DOI] [PubMed] [Google Scholar]
- 43.Johnson JD, Rugg MD. Modulation of the electrophysiological correlates of retrieval cue processing by the specificity of task demands. Brain Research. 2006;1071:153–164. doi: 10.1016/j.brainres.2005.11.093. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MK, Kounios J, Nolde S. Electrophysiological brain activity and source monitoring. Neuroreport. 1997;8:1317–1320. doi: 10.1097/00001756-199703240-00051. [DOI] [PubMed] [Google Scholar]
- 45.Johnson MK, Kounios J, Reeder JA. Time-course studies of reality monitoring and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1409–1419. doi: 10.1037//0278-7393.20.6.1409. [DOI] [PubMed] [Google Scholar]
- 46.Karlsson T, Bäckman L, Herlitz A, Nilsson LG, Winblad B, Oserlind PO. Memory improvement at different stages of Alzheimer's Disease. Neuropsychologia. 1989;27:737–742. doi: 10.1016/0028-3932(89)90119-x. [DOI] [PubMed] [Google Scholar]
- 47.Kazmerski VA, Friedman D. Old/new differences in direct and indirect memory tests using pictures and words in within- and cross-form conditions: event-related potential and behavioral measures. Cognitive Brain Research. 1997;5:255–272. doi: 10.1016/s0926-6410(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 48.Knopf M. Having shaved a kiwi: Memory for unfamiliar subject-performed actions. Psychological Research. 1991;53:203–211. [Google Scholar]
- 49.Knopf M, Neidhardt E. Aging and memory for action events: The role of familiarity. Developmental Psychology. 1989;25:780–786. [Google Scholar]
- 50.Koriat A, Ben-Zur H, Druch A. The contextualization of input and output events in memory. Psychological Research. 1991;53:260–270. doi: 10.1007/s004260050034. [DOI] [PubMed] [Google Scholar]
- 51.Kormi-Nouri R, Nyberg L, Nilsson LG. The effects of retrieval enactment on recall of subject-performed and verbal tasks. Memory and Cognition. 1994;22:723–728. doi: 10.3758/bf03209257. [DOI] [PubMed] [Google Scholar]
- 52.Kormi-Nouri R, Nilsson LG. The motor component is not crucial! In: Zimmer HD, Cohen RL, Guynn MJ, Engelkamp J, Kormi-Nouri R, Foley MA, editors. Memory for Action. A Distinct Form of Episodic Memory? Oxford: Oxford University Press; 2001. pp. 97–111. [Google Scholar]
- 53.Kuo TY, Van Petten C. Prefrontal engagement during source memory retrieval depends on the prior encoding task. Journal of Cognitive Neuroscience. 2006;18:1133–1146. doi: 10.1162/jocn.2006.18.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leynes PA, Cairns A, Crawford JT. Event-related potentials indicate that reality monitoring differs from external source monitoring. American Journal of Psychology. 2005;118:497–524. [PubMed] [Google Scholar]
- 55.Lindsay DS, Johnson MK. The eyewitness suggestibility effect and memory for source. Memory and Cognition. 1989;17:349–358. doi: 10.3758/bf03198473. [DOI] [PubMed] [Google Scholar]
- 56.McAndrews MP, Milner B. The frontal cortex and memory for temporal order. Neuropsychologia. 1991;29:849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- 57.McCarthy G, Woods CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 58.McClelland JM, Mcnaughton BL, O'reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;109:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 59.Mimura M, Komatsu S, Kato M, Yashimasu H, et al. Memory for subject performed tasks in patients with Korsakoff syndrome. Cortex. 1998;34:297–303. doi: 10.1016/s0010-9452(08)70757-3. [DOI] [PubMed] [Google Scholar]
- 60.Mohr G, Engelkamp J, Zimmer HD. Recall and recognition of self-performed acts. Psychological Research. 1989;51:181–187. [Google Scholar]
- 61.Mulligan NW. Generation and hypermnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:436–450. doi: 10.1037/0278-7393.27.2.436. [DOI] [PubMed] [Google Scholar]
- 62.Nelson DL, Canas JJ, Bajo MT, Keelean PD. Comparing word fragment completion and cued-recall with letter cues. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:542–552. doi: 10.1037//0278-7393.13.4.542. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson LG, Craik FIM. Additive and interactive effects in memory for subject-performed tasks. European Journal of Cognitive Psychology. 1990;2:305–324. [Google Scholar]
- 64.Nilsson LG, Nyberg L, Klingberg T, Åberg C, Persson J, Roland PE. Activity in motor areas while remembering action events. Neuroreport. 2000;11:2199–2101. doi: 10.1097/00001756-200007140-00027. [DOI] [PubMed] [Google Scholar]
- 65.Norris MP, West RL. Age differences in the recall of actions and cognitive activities. Psychological Research. 1991;53:188–194. doi: 10.1007/BF00941386. [DOI] [PubMed] [Google Scholar]
- 66.Nyberg L, Nilsson LG. The role of enactment in implicit and explicit memory. Psychological Research. 1995;57:215–219. doi: 10.1007/BF00431282. [DOI] [PubMed] [Google Scholar]
- 67.Nyberg L, Petersson KM, Nilsson LG, Sandblom J, Aberg C, Ingvar M. Reactivation of motor brain areas during explicit memory for actions. Neuroimage. 2001;14:521–528. doi: 10.1006/nimg.2001.0801. [DOI] [PubMed] [Google Scholar]
- 68.Olichney J, Van Petten C, Paller K, Salmon D, Iragui V, Kutas M. Word repetition in amnesia: Electrophysiological evidence of spared and impaired memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- 69.Paller KA. Consolidating dispersed neocortical memories: The missing link in amnesia. Memory. 1997;5:73–88. doi: 10.1080/741941150. [DOI] [PubMed] [Google Scholar]
- 70.Peynircioglu ZF. The generation effect with pictures and nonsense figures. Acta Psychologica. 1989;70:153–160. [Google Scholar]
- 71.Rahal TA, May CP, Hasher L. Truth and character: Sources that older adults can remember. Psychological Science. 2002;13:101–105. doi: 10.1111/1467-9280.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranganath C, Paller KA. Neural correlates of memory retrieval and evaluation. Cognitive Brain Research. 2000;9:209–222. doi: 10.1016/s0926-6410(99)00048-8. [DOI] [PubMed] [Google Scholar]
- 73.Raye CL, Johnson MK, Mitchell KJ. FMRI investigations of left and right PFC contributions to episodic remembering. Psychobiology. 2000;28:197–206. [Google Scholar]
- 74.Rizzolatti G, Craighero L, Fadiga L. The mirror neuron system in humans. In: Stamenov MI, Gallese V, editors. Mirror neurons and the evolution of brain and language. Amsterdam: John Benjamins; 2002. pp. 37–62. [Google Scholar]
- 75.Robb WG, Rugg MD. Electrophysiological dissociation of retrieval orientation and retrieval effort. Psychonomic Bulletin and Review. 2002;9:583–589. doi: 10.3758/bf03196316. [DOI] [PubMed] [Google Scholar]
- 76.Roediger HL, III, Weldon MS, Challis BH. Explaining dissociations between implicit and explicit measures of retention: A processing account. In: Roediger HL III, Craik FIM, editors. Varieties of memory and consciousness. Essays in honour of Endel Tulving. Hillsdale N.J.: Erlbaum; 1989. pp. 3–42. [Google Scholar]
- 77.Rubin SR, Van Petten C, Glisky EL, Newberg W. Memory conjunction errors in younger and older adults: Event-related potential and neuropsychological data. Cognitive Neuropsychology. 1999;16:459–488. [Google Scholar]
- 78.Rugg MD, Fletcher PC, Chua PML, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- 79.Schendan HE, Kutas M. Neurophysiological evidence for two processing times for visual object identification. Neuropsychologia. 2002;40:931–945. doi: 10.1016/s0028-3932(01)00176-2. [DOI] [PubMed] [Google Scholar]
- 80.Schendan HE, Kutas M. Time course of processes and representations supporting visual object identification and memory. Journal of Cognitive Neuroscience. 2003;15:111–135. doi: 10.1162/089892903321107864. [DOI] [PubMed] [Google Scholar]
- 81.Senkfor AJ. To have and to hold: Memory for pantomimed actions versus actions with real objects. doi: 10.1016/j.cortex.2007.03.003. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Senkfor AJ. Episodic action memory: Characterization of the time course and neural circuitry. In: Stamenov M, Gallese V, editors. Mirror Neurons and the Evolution of the Brain. John Benjamins Publishing Co; Amsterdam: 2002. [Google Scholar]
- 83.Senkfor AJ, Van Petten C. Who said what? An event-related potentials investigation of source and item memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- 84.Senkfor AJ, Van Petten C, Kutas M. Episodic action monitoring: An ERP study with perform, watch, and imagine action encoding tasks versus an non-action task. Journal of Cognitive Neuroscience. 2002;14:402–439. doi: 10.1162/089892902317361921. [DOI] [PubMed] [Google Scholar]
- 85.Slamecka NJ, Graf P. The generation effect: Delineation of a phenomena. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1978;4:592–604. [Google Scholar]
- 86.Stenberg G, Johansson M, Rosén I. Conceptual and perceptual memory: Retrieval orientations reflected in event-related potentials. Acta Psychologica. 2006;122:174–205. doi: 10.1016/j.actpsy.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Svenson T, Nilsson LG. The relationship between recognition and cued-recall in memory for enacted and nonenacted information. Psychological Research. 1989;51:194–200. [Google Scholar]
- 88.Trott CT, Friedman D, Ritter W, Fabiani M. Item and source memory: Differential age effects revealed by event-related potentials. Neuroreport. 1997;8:3373–3378. doi: 10.1097/00001756-199710200-00036. [DOI] [PubMed] [Google Scholar]
- 89.Van Petten C, Luka BJ, Rubin SR, Ryan JP. Frontal brain activity predicts individual performance in an associative memory exclusion task. Cerebral Cortex. 2002;12:1180–1192. doi: 10.1093/cercor/12.11.1180. [DOI] [PubMed] [Google Scholar]
- 90.Van Petten C, Senkfor AJ. Memory for words and novel visual patterns: Repetition, recognition, and encoding effects in the event-related potential. Psychophysiology. 1996;33:491–506. doi: 10.1111/j.1469-8986.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 91.Van Petten C, Senkfor AJ, Newberg W. Memory for drawings in locations: Spatial source memory and event-related potentials. Psychophysiology. 2000;37:551–564. [PubMed] [Google Scholar]
- 92.Warrington EK, Ackroyd C. The effect of orienting task on recognition memory. Memory and Cognition. 1975;3:140–142. doi: 10.3758/BF03212890. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe H. Effects of encoding style: Expectations of retrieval mode, and retrieval style on memory for action phrases. Perceptual and Motor Skills. 2003;96:707–727. doi: 10.2466/pms.2003.96.3.707. [DOI] [PubMed] [Google Scholar]
- 94.Wilding EL. Separating retrieval strategies from retrieval success: an event-related potential study of source memory. Neuropsychologia. 1999;37:441–454. doi: 10.1016/s0028-3932(98)00100-6. [DOI] [PubMed] [Google Scholar]
- 95.Wilding EL, Doyle MC, Rugg MD. Recognition memory with and without retrieval context: An event-related potential study. Neuropsychologia. 1995;33:743–767. doi: 10.1016/0028-3932(95)00017-w. [DOI] [PubMed] [Google Scholar]
- 96.Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- 97.Zaragoza MS, Koshmider JW., 3rd Misled subjects may know more than they performance implies. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:246–255. doi: 10.1037//0278-7393.15.2.246. [DOI] [PubMed] [Google Scholar]
- 98.Zaragoza MS, Lane SM. Source misattributions and the suggestibility of eyewitness testimony. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:934–945. doi: 10.1037//0278-7393.20.4.934. [DOI] [PubMed] [Google Scholar]
- 99.Zimmer HD. Why do actions speaker louder than words?: Action memory as a variant of encoding manipulations or as a result of a specific memory system? In: Zimmer HD, Cohen RL, Guynn MJ, Engelkamp J, Kormi-Nouri R, Foley MA, editors. Memory for Action A Distinct Form of Episodic Memory? Oxford: Oxford University Press; 2001. pp. 151–198. [Google Scholar]
- 100.Zimmer HD, Engelkamp J. Levels-of-processing effects in subject-performed tasks. Memory and Cognition. 1999;27:907–914. doi: 10.3758/bf03198543. [DOI] [PubMed] [Google Scholar]
- 101.Zimmer HD, Helstrup T, Engelkamp J. Pop-out into memory: A retrieval mechanism that is enhanced with the recall of subject-performed tasks. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26:658–680. doi: 10.1037//0278-7393.26.3.658. [DOI] [PubMed] [Google Scholar]


