Figure 5. EGFR Interacts with and Stabilizes SGLT1 Independent of EGFR Kinase Activity.
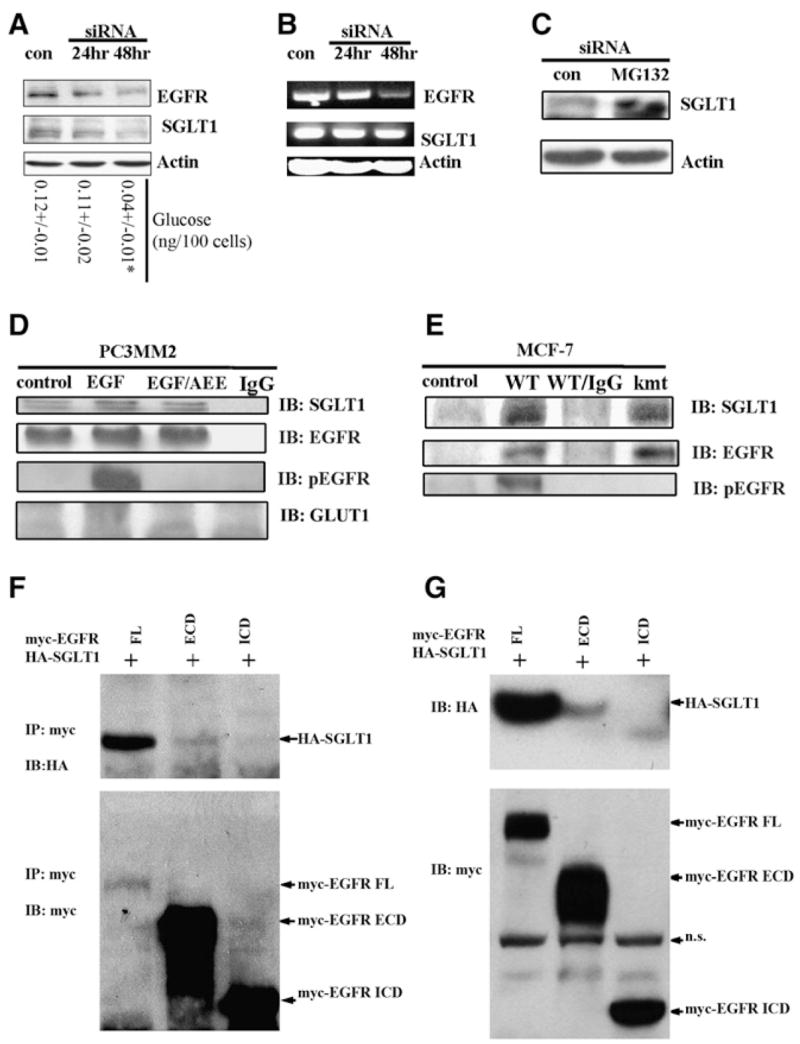
(A) Western blot revealed a time-dependent downregulation of SGLT1 by knocking down EGFR with siRNA (con, control). At each time point, levels of glucose were measured. *p < 0.05.
(B) RT-PCR analysis of EGFR mRNA and SGLT1 mRNA in response to EGFR siRNA treatment (con, control). The EGFR mRNA became downregulated at 48 hr after EGFR siRNA treatment, whereas the SGLT1 mRNA level remained unchanged.
(C) Proteasome inhibitor MG132 blocked the downregulation of SGLT1 by EGFR siRNA; siRNA and MG132 treatments were for 24 hr. Actin was used as an internal control.
(D) EGFR physically interacted with SGLT1, independent of EGFR kinase activity, as revealed by coimmunoprecipitation with the anti-EGFR antibody C225. PC3MM2 cells were cultured in serum-free medium for 12 hr before being treated with 40 ng/ml EGF or with EGF and AEE788 (EGF/AEE). The blotting of immunoprecipitates was against SGLT1, total EGFR, pEGFR, and GLUT1. Normal mouse IgG was used as a negative control).
(E) kmtEGFR also interacted with SGLT1 as did WT-EGFR in MCF-7 cells co-transfected with WT-EGFR/SGLT1 or kmtEGFR/SGLT1.
(F) HA-SGLT1 was coimmunoprecipitated with myc-EGFR and myc-ECD, but not myc-ICD.
(G) Western blotting detection of HA-SGLT1 coexpressed with myc-tagged full-length (FL), the extracellular domain (ECD containing the transmembrane domain), and intracellular domain (ICD) of EGFR in HEK293 cells. Note that HA-SGLT1 was only efficiently coexpressed with full-length EGFR, to much less extent with ECD, but not with ICD of EGFR (IP, immunoprecipitation; IB, immunoblotting; n.s., nonspecific band).
