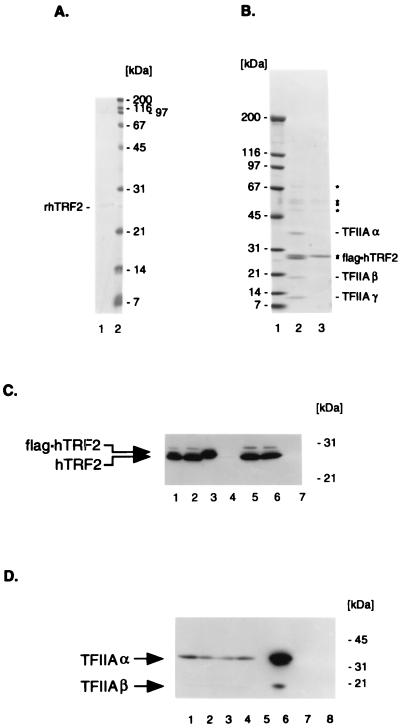
Figure 3.
Purification of hTRF2 and associated proteins. (A) SDS/PAGE (15%) analysis of purified rhTRF2. Lane 1: 1 M KCl eluate of an EMD-SO3−-Fractogel column containing 50 ng of His-tagged rhTRF2. Lane 2: Molecular weight marker with masses (in kDa at right). The gel was stained with Coomassie Brilliant Blue. (B) SDS/PAGE (5–20%) analysis of purified flag⋅hTRF2–TFIIA complex. Lane 1: Molecular weight markers with masses at the left. Lane 2: The flag⋅hTRF2–TFIIA complex purified from 1 ml of TRF4–1 nuclear extract over M2-Agarose and eluted with FLAG peptide; positions of flag-tagged TRF and associated α, β, and γ subunits of TFIIA (at the right). Lane 3: Proteins from control HeLa S extracts which are bound to M2 agarose and eluted with FLAG peptide under identical conditions; asterisks indicate major nonspecific proteins. The gel was stained with Coomassie Brilliant Blue. (C) Western blot analysis with anti-hTRF2 serum. Lanes 1 and 5: 30 μg of nuclear extract from TRF4–1 and HeLa S cell lines, respectively. Lanes 2 and 6: 30 μg of M2 Agarose flowthrough fractions from TRF4–1 and HeLa S nuclear extracts, respectively. Lanes 3 and 7: FLAG peptide-eluted M2 agarose fractions from TRF4–1 and HeLa S nuclear extracts, respectively. Lane 4: Molecular weight markers. Migration of flag⋅hTRF2 and hTRF2 is shown on the left and positions of molecular weight markers are on the right. SDS/PAGE and Western blot assays were essentially as described (36). (D) Western blot analysis with anti-TFIIAα/β (p55) antibodies. Lanes 1 and 2: 30 μg of nuclear extracts from TRF4–1 and HeLa S cells, respectively. Lanes 3 and 4: 30 μg of M2 agarose flowthrough fraction from TRF4–1 and from HeLa S nuclear extracts, respectively. Lanes 6 and 8: FLAG peptide M2 agarose eluates from TRF4–1 and HeLa S nuclear extracts, respectively. Lanes 5 and 7: Mixtures of molecular weight marker proteins. The migration of TFIIAα and TFIIAβ, as well as of molecular weight marker proteins, is indicated.