Abstract
Procurement of donor pancreases for islet isolation and transplantation is not yet widely practiced due to concerns about post-mortem ischemia upon functional islet yields. Perfusion/preservation technology can help to circumvent ischemic injury and is applied in this study to porcine pancreata (Px) prior to islet isolation. Px harvested from adult pigs were assigned to one of three preservation treatment groups:G1) Fresh controls - processed immediately with minimum cold ischemia(<1h) G2) Static Cold Storage-flushed with cold UW-Viaspan and stored at 2–4ºC for 24h, and G3) Hypothermic Machine Perfusion (HMP)-perfused on a pulsatile LifePort® machine with KPS1 solution at 4–7ºC and low pressure(10mmHg) for 24h. Islet isolation was then accomplished using conventional methods and standard accepted product release criteria were used to assess islet yield and function. Islet yield was markedly different between the treatment groups and the increased yield in the HMP group over the static cold storage in UW-Viaspan was statistically significant (p<0.05). Functionally, the islets from each experimental group were equivalent and not significantly different to fresh controls (G1). Dithizone staining for islets showed a consistently more uniform digestion of the Px from G3 compared with G1 and G2, with greater separation of the tissue and less entrapped islets. HMP for 24h is well tolerated leading to moderate edema but no loss of function of the harvested islets. The edema appears to aid in enzymatic digestion producing a greater yield and purity of islets compared with Px subjected to 24h of static cold storage.
Introduction
Implantation of functional islet cells is a potential cure for diabetes but the availability of high quality islets for transplantation is critical for success. Procurement of donor pancreases for islet isolation and transplantation is not yet widely practiced due in part to concerns about post-mortem ischemia upon functional islet yields. Perfusion/preservation technology has had a major impact in circumventing ischemic injury in kidney transplantation [1–3]. Here we applied this approach to the preservation and procurement of viable islets after hypothermic perfusion preservation of porcine pancreata.
Methods
Using anesthetized pigs (Domestic Yorkshire, 25–30 kg), pancreases were flushed in situ with cold Lactated Ringer’s solution (2L) prior to excision using a surgical technique that preserved the head, tail and body of the pancreas together with a section of the duodenum to protect the pancreaticoduodenal arteries. For ex vivo flushing and perfusion, the superior mesenteric artery and celiac trunk were cannulated and the splenic vessels and all arterial branches on the margin of gastroduodenal, splenic and hepatic sides of the pancreas were ligated to allow uniform perfusion through the gland with effluent flow through the portal vein. Pancreases were assigned to one of three experimental groups in which they were processed for islet isolation after the following preservation treatments: G1) Fresh controls – processed immediately with minimum cold ischemia (<2h) [n=7]; G2) Static Cold Storage - flushed with cold UW-Viaspan and stored at 2–4°C for 24h [n=9], and G3) Hypothermic Machine Perfusion (HMP) – perfused on a pulsatile LifePort® machine (Organ Recovery Systems, Inc. Des Plaines, IL) with KPS1 solution (Organ Recovery Systems, Inc. Des Plaines, IL.) at 4–7°C and low pressure (10mmHg) for 24h [n=7]. At the end of the perfusion interval the pancreas was removed from the LifePort perfusion machine and processed for islet isolation. This was accomplished using standard techniques involving ductal distension of the gland with liberase (PI) enzyme (Roche, porcine), normothermic digestion and density gradient purification (Ficoll continuous density gradient on a COBE 2991 (Gambro BCT) cell separator) [4]. Standard, accepted product release criteria recently adopted by the major clinical centers were implemented in this study. These tests included islet quantification, islet viability and histology, and functional viability assessment using the glucose stimulated insulin secretion assay [5].
Results and Discussion
Islet retrieval data
Progressive edema during continuous hypothermic perfusion of organs is an intrinsic event that is usually restricted by optimizing perfusion media and conditions [6–8]. In this study the measured degree of edema was 0%; −2.8±0.7%; and 138±19% for the groups respectively (Table 1). Negative edema in G2 was due to the hypertonicity of UW-Viaspan during static cold storage. Islet yield expressed as Islet Equivalents (IEQ) was markedly different between the treatment groups: G1=1306 ± 348IEQ/g; G2=784±181 IEQ/g; G3=2397±478 IEQ/g. The increased yield in the HMP group over the static cold storage in UW-Viaspan was statistically significant (p<0.05). The purity of the islet preps, measured as the ratio of insulin (from islets) to amylase (from exocrine cells), was: G1= 4.7±1.1%; G2=11.5±2%; G3=25.5±5%. Microscopic examination of the different preparations using Dithizone staining for islets showed a consistently more uniform digestion of the Px from G3 compared with G1 and G2, with greater separation of the tissue and less entrapped islets. It was observed throughout the study that the method of preservation had a significant impact on the extent of digestion time and the amount of free islets released from the pancreatic digest. Islet sampling during the process of digestion revealed early free islets and a more homogenous digest, without fragments of exocrine tissue, for the machine perfused pancreata. Tissue digest from both fresh and control group pancreata showed more mantled (incompletely cleaved islets with adherent exocrine tissue) and entrapped islets in comparison to perfused organs.
Table 1.
Islet Yield and Function Data.
| PANCREAS/ISLET CHARACTERISTICS | G1:FRESH (untreated control) [N=7] | G2:CONTROL (Viaspan) [N=9] | G3:HMP (KPS-1) [N=7] |
|---|---|---|---|
| Pancreas weight [g] | 114.60±7.16 | 118.10±5.18 | 107.10±8.03 |
| Post–Preservation Edema [%] | - | −2.79±0.71 | 138±18.6 |
| Digested Tissue [%] | 82.62±2.62 | 81.38±1.97 | 76.13±3.53 |
| Digestion time [s] | 757±61 | 707±39 | 638±27 |
| Total Islet Equivalents [IE] | 116,894±31,428 | 74,956.9±16,396 | 187,857±36,608+ |
| IE/(g of digested tissue) | 1305±348 | 784±181 ** | 2397±478 |
| Insulin Stimulation Index | 4.59±1.33 | 2.45±0.37 | 2.88±0.44 |
| High-glucose insulin [ng/mL/IE] | 0.33±0.15 | 0.20±0.05 | 0.23±0.08 |
| Insulin content [ng/mL/IE] | 8.51±3.68 | 4.75±1.00 | 11.80±3.79# |
| Amylase content [μg/mL] | 102.13±59.10 | 13.20±2.14 | 28.2±7.33 |
| Insulin/Amylase [%] | 4.71±1.13*** | 11.54±1.89 | 25.53±5.02 |
p<0.05 vs. G2:Control group;
p<0.05 vs. G3:HMP.
p<0.05 vs. G3:HMP;
p=<0.05 vs. G2:Control group.
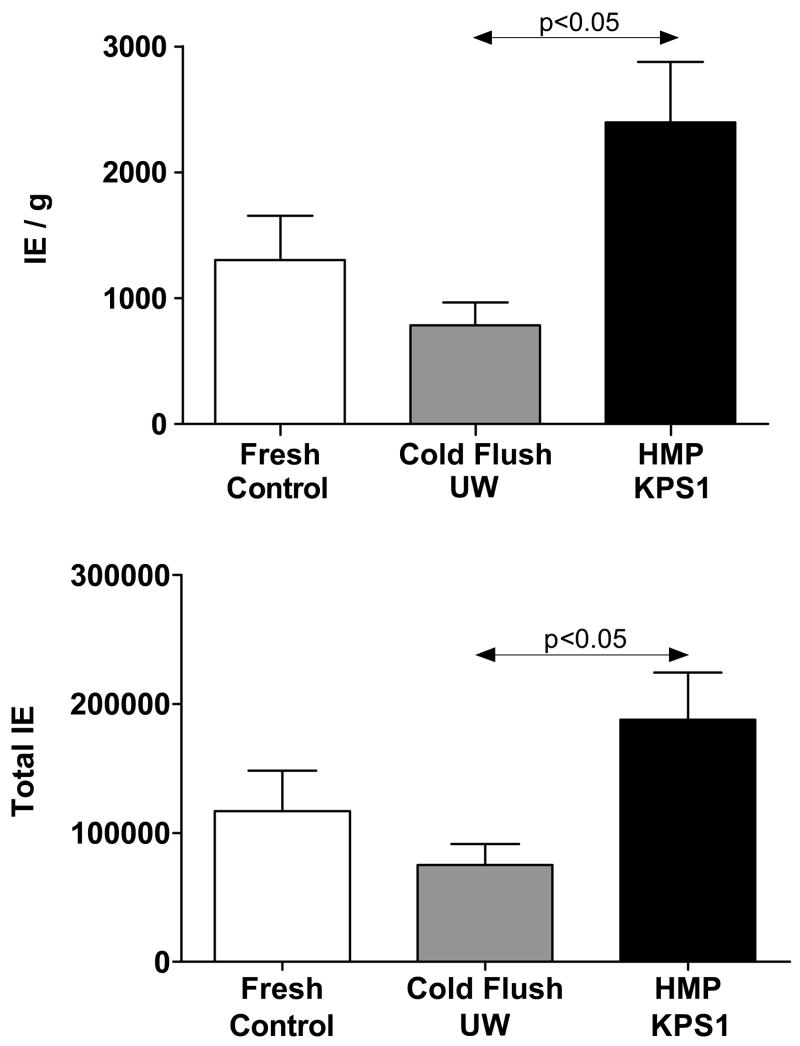
The islet retrieval data is summarized in Fig. 1, which shows that pancreas perfusion resulted in statistically significantly higher IE per gram of digested tissue when compared to the fresh and control groups. Machine perfusion allowed the remnant blood to be washed off and also, based on the amount of water accumulation (edema) provided a disrupted extracellular space without a negative impact on the ductal distension and islet viability. These ultimately helped rapidly free more islets and a correlation between edema and digestion time seems to exist (638±26 sec and 138±19 % edema for HMP and 757±61 sec and 707±38sec and no edema for Fresh (G1) and cold stored in UW (G2), respectively). The greater the edema the shorter the digestion time.
Figure 1.
Islet retrieval data (Mean ± SEM) expressed as both Islet Equivalents (IE) per unit weight of digested pancreas (UPPER GRAPH) and total islet yield per pancreas (LOWER GRAPH). Group 1: Fresh Control Pancreases (N=7); Group 2: Preservation controls cold stored in UW solution (N=9); Group 3: Hypothermic Machine Perfused (HMP) with KPS1 solution (N=7).
Islet Integrity
Table 1 summarizes the data for islet function in terms of insulin content and the ability to respond to a secretory challenge. The latter is expressed as the Stimulation Index determined by comparing the insulin released during sequential exposure to a low (2mM, non-stimulatory) and high (20mM, stimulatory) concentration of glucose. Functionally, the islets from each experimental group were equivalent and not significantly different to controls (G1) with insulin stimulation indices of G1=4.6±1.3; G2=2.5±0.4; G3=2.9±0.4. However, insulin content (ng/ml/IEQ) was different between the treatment groups with the highest insulin content in islets harvested from HMP Px (G3)=11.8±3.8 compared with G1=8.5±3.7 and G2=4.8±1.1 for fresh controls and static cold storage respectively. The mean insulin content of islets isolated from perfused pancreata (G3) was significantly higher than that of the UW/Viaspan cold stored control group (G2) and was not significantly different to the mean values from fresh tissue (G1). Moreover, the stimulation indices showed that the insulin secretory function of the islets isolated from perfused pancreata was not compromised when compared with the control groups.
Histology Results
The basic structure of the perfused pancreases was well preserved showing the normal conformation of secretory cells with an abundance of secretory granules concentrated at the apical pole of the pyramidal cells. By contrast, some moderate degree of cellular disruption and degranulation was observed in samples processed from pancreases cold stored for 24h in UW-Viaspan.
Ever since the first experimental attempts to ameliorate Type I diabetes by transplantation of allograft donor islets the field has been challenged by the need for improved methods of retrieving islets from donor pancreata. During the course of these studies we discovered that the technique of hypothermic machine perfusion preservation resulted in a greater yield of islets than either fresh, or static cold stored pancreases. In fact, the increased yield over the fresh group of pancreases was 1.6–1.8 times greater, and approximately 3-fold greater than for pancreases preserved in UW/Viapsan.
We conclude that 24h of HMP is well tolerated leading to moderate edema but no loss of function of the harvested islets. On the contrary, the edema appears to aid in the subsequent breakup of the gland during enzymatic digestion producing a greater yield and purity of islets compared with pancreases subjected to 24h of static cold storage in UW-Viaspan. Further research is needed to determine whether this phenomenon is peculiar to the porcine model, or whether the same salutary effect of hypothermic machine perfusion on islet isolation can be achieved with human pancreases.
Acknowledgments
We gratefully acknowledge Dr Horacio Rilo (University of Cincinnati) for advice and assistance in establishing the islet isolation technique for large animal pancreases in our laboratory.
Funding: This study was funded in part by a research grant from the National Institutes of Health (5R44DK065508-03)
References
- 1.Daemen JHC, DeVries B, Oomen APA, DeMeester J, Kootstra G. Effect of machine perfusion preservation on delayed graft function in non-heart beating donor kidneys- early results. Transpl Int. 1997;10:317–322. doi: 10.1007/s001470050063. [DOI] [PubMed] [Google Scholar]
- 2.Koyama H, Cecka JM, Terasaki PI. A comparison of cadaver kidney storage methods: pump perfusion and cold storage solutions. Clin Transplant. 1993;7:199–205. [Google Scholar]
- 3.Merion RM, Oh HK, Port FK, Toledo-Pereyra LH, Turcotte JG. A prospective controlled trial of cold-storage versus machine-perfusion preservation in cadaveric renal transplantation. Transplantation. 1990;50:230–233. doi: 10.1097/00007890-199008000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Lakey JRT, Kobayashi N, Shapiro AMJ, Ricordi C, Okitsu T. Current Human Islet Isolation Protocol. Chuoku, Osaka: Medical Review Co. Ltd; 2004. [Google Scholar]
- 5.Ricordi C, Hering B, London NJ, Rajotte RV, Gray DWR, Socci C, Alejandro R, Carroll PB, Bretzel RG, Scharp DW. Islet Isolation Assessment. In: Ricordi C, editor. Pancreatic Islet Cell Transplantation. Austin: R.G. Landes; 1992. pp. 132–142. [Google Scholar]
- 6.Taylor MJ. Biology of cell survival in the cold: The Basis for Biopreservation of Tissues and Organs. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Boca Raton: CRC Press; 2007. pp. 15–62. [Google Scholar]
- 7.Brockbank KG, Taylor MJ. Tissue Preservation. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Boca Raton: CRC Press; 2007. pp. 157–196. [Google Scholar]
- 8.Hafez T, Fuller B. Applications: Organ Preservation for Transplantation. In: Baust John G, Baust John M., editors. Advances in Biopreservation. Boca Raton: Taylor & Francis; 2007. pp. 197–270. [Google Scholar]