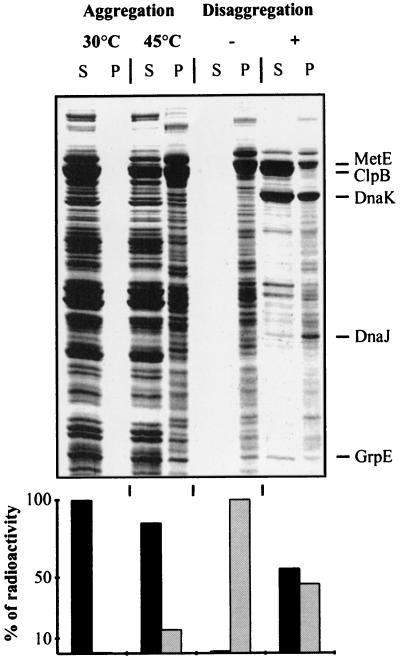
Figure 4.
Solubilization of a wide range of heat-aggregated proteins. Soluble 35S-labeled protein extracts from wild-type E. coli cells (MC4100) were aggregated at 45°C and incubated for 4 hr with ATP, ClpB, and KJE (see Experimental Procedures). Resolubilized and aggregated proteins were separated by centrifugation. Gels were loaded with equal volumes, such that the insoluble fractions were 2.5-fold more concentrated relative to the soluble fractions. Soluble (S) and aggregated (P) proteins were analyzed by Coomassie staining (Upper) and quantified by scintillation counting (Lower).