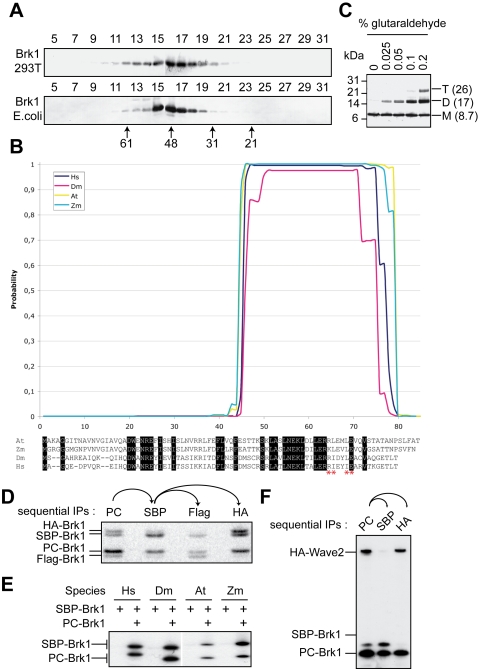
Figure 3. Free Brk1 is a trimer.
A Partially purified untagged Brk1 expressed in 293T cells, and purified Brk1 expressed in E.coli, were analyzed by Size Exclusion Chromatography (SEC) using a superdex-200 column and western blotting. The numbered lanes refer to elution fractions. Stokes' radii of standards are given in Å. B Prediction of a coiled-coil in the carboxy-terminal half of Brk1 from different species (Hs: Homo sapiens, Dm: Drosophila melanogaster, At: Arabidopsis thaliana, Zm: Zea mays). In reverse lettering are indicated conserved residues in all four species. Red stars indicate the signature for trimeric coiled coils, R-h1-x-x-h2-E, where x is any amino-acid, h1 is I, L, V, or M and h2 is I, L, or V. C Free Brk1 was analyzed by western blotting after glutaraldehyde cross-linking at the indicated concentrations. The position of molecular weight markers is indicated on the left. Monomers (M), dimers (D) and trimers (T) are indicated with their calculated mass on the right. D A mixture of PC-, SBP-, Flag- and HA-Brk1 obtained by In Vitro Translation (IVT) was immunoprecipitated sequentially as indicated. Complexes containing at least one PC- and one SBP-Brk1 were selected by two sequential immunoprecipitations as indicated. As a third step, either Flag- or HA-Brk1 was immunoprecipitated. After three sequential immunoprecipitations, the signals for all three selected subunits are similar. This indicates that the oligomer is a trimer composed of one of each selected form of Brk1. E Oligomerization is a conserved property of Brk1. Oligomerization of Brk1 from different species (Hs: Homo sapiens; Dm: Drosophila melanogaster; At: Arabidopsis thaliana; Zm: Zea mays) was assessed by the co-translation of PC-Brk1 and SBP-Brk1 as above, and the sequential immunoprecipitations of PC-Brk1 and then of SBP-Brk1. The selection of complexes containing at least one PC- and one SBP-Brk1 in all four species indicates that Brk1 oligomerizes in all tested species, most likely trimerizes as demonstrated above for the human protein. F Wave2 binds to a single molecule of Brk1. A mixture of PC- Brk1, SBP-Brk1 and HA-Wave2 obtained by IVT was immunoprecipitated sequentially as indicated. A single PC immunoprecipitation retrieves both the trimers and Wave. SBP precipitation, as a second step, selects trimers containing both PC and SBP-Brk1, but excludes Wave2. Conversely, HA immunoprecipitation, as a second step, retrieves Wave2 bound to PC-Brk1, but excludes SBP-Brk1. Thus Brk1 does not bind to Wave2 as an oligomer.