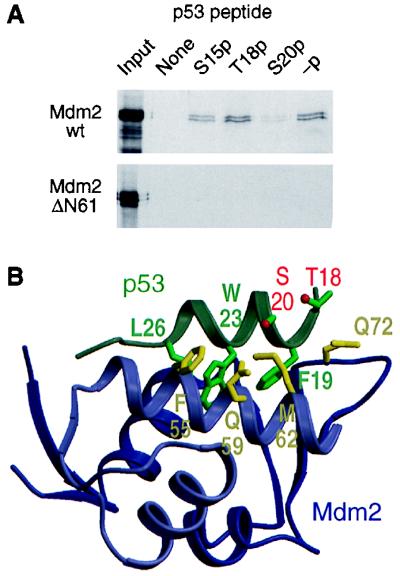
Figure 5.
Effect of phosphorylation of Ser-20 on the interaction of p53 with Mdm2. (A) Capture of 35S-labeled in vitro-translated full-length or N-terminally truncated (ΔN61) Mdm2 by phosphorylated and nonphosphorylated p53 peptides corresponding to residues 7–29 of human p53; -p, nonphosphorylated peptide; S15p, T18p, and S20p, peptides phosphorylated on Ser-15, Thr-18, and Ser-20, respectively. (B) Three-dimensional structure of the p53–Mdm2 complex (PDB ID code 1YCR). The p53 peptide shown (residues 17–29) is the entire p53 region that associates with Mdm2. Selected amino acid side chains of p53 and Mdm2 are shown and labeled by using the codon number and single-letter residue code: F, Phe; L, Leu; M, Met; Q, Gln; S, Ser; T, Thr; and W, Trp. The oxygen atoms of the hydroxyl groups of Thr-18 and Ser-20 are colored red. The figure was prepared by using molscript (58) and raster3d (59).